
A SOP template for data entry and management provides a structured framework to ensure consistency, accuracy, and efficiency in handling data tasks. It outlines step-by-step procedures for inputting, verifying, and organizing information, which minimizes errors and enhances data integrity. Following this template supports compliance with organizational standards and improves overall data workflow management.
Standard procedure for data collection and entry.

This SOP defines the standard procedure for data collection and entry, covering methods for accurate data gathering, validation processes, proper documentation, and systematic data input into designated databases or software systems. The goal is to ensure data integrity, consistency, and reliability throughout all phases of collection and entry to support effective decision-making and reporting.
Data validation and quality assurance steps.

This SOP details the data validation and quality assurance steps necessary to ensure accuracy, consistency, and reliability of data throughout its lifecycle. It covers procedures for data entry validation, automated checks, manual reviews, error detection and correction, quality control audits, and documentation standards. The goal is to maintain high-quality data that supports effective decision-making and operational efficiency.
User access control and authorization protocols.

This SOP defines user access control and authorization protocols, including user identification and authentication processes, role-based access management, permission assignment and review, multi-factor authentication implementation, access monitoring and logging, password policies and management, and procedures for access revocation and privilege escalation prevention. The objective is to secure system resources by ensuring only authorized users gain appropriate access according to their roles and responsibilities, thereby protecting sensitive data and maintaining compliance with security policies.
Data entry software and tools usage guidelines.

This SOP provides comprehensive data entry software and tools usage guidelines to ensure accurate, efficient, and secure data input practices. It covers proper software selection, user access and permissions, standardized data entry procedures, data validation and error-checking techniques, regular software updates and maintenance protocols, backup and data recovery processes, and training requirements for all users. The objective is to maintain data integrity, enhance productivity, and minimize errors throughout data management operations.
File naming conventions and version control.

This SOP defines file naming conventions and version control to ensure consistent, clear, and organized management of digital documents and files. It includes guidelines for standardized file names incorporating project details, dates, and version numbers, procedures for tracking file revisions, and best practices for collaborative editing and archiving. The goal is to enhance file accessibility, prevent duplication, and maintain accurate version histories across teams.
Backup and data storage procedures.

This SOP defines backup and data storage procedures to ensure the security, integrity, and availability of critical data. It covers protocols for regular data backups, selection of appropriate storage media, data encryption and access controls, retention schedules, verification and testing of backup copies, and disaster recovery planning. The purpose is to minimize data loss risks and maintain business continuity through systematic and reliable data management practices.
Error detection and correction workflow.

This SOP defines the error detection and correction workflow, detailing a structured process for identifying, reporting, analyzing, and resolving errors in data or system operations. It includes methods for automated and manual error detection, classification of error types, prioritization for correction, implementation of corrective actions, validation of fixes, and documentation of error resolution steps. The goal is to ensure data integrity, system reliability, and continuous improvement through timely and accurate error management.
Data privacy and confidentiality measures.
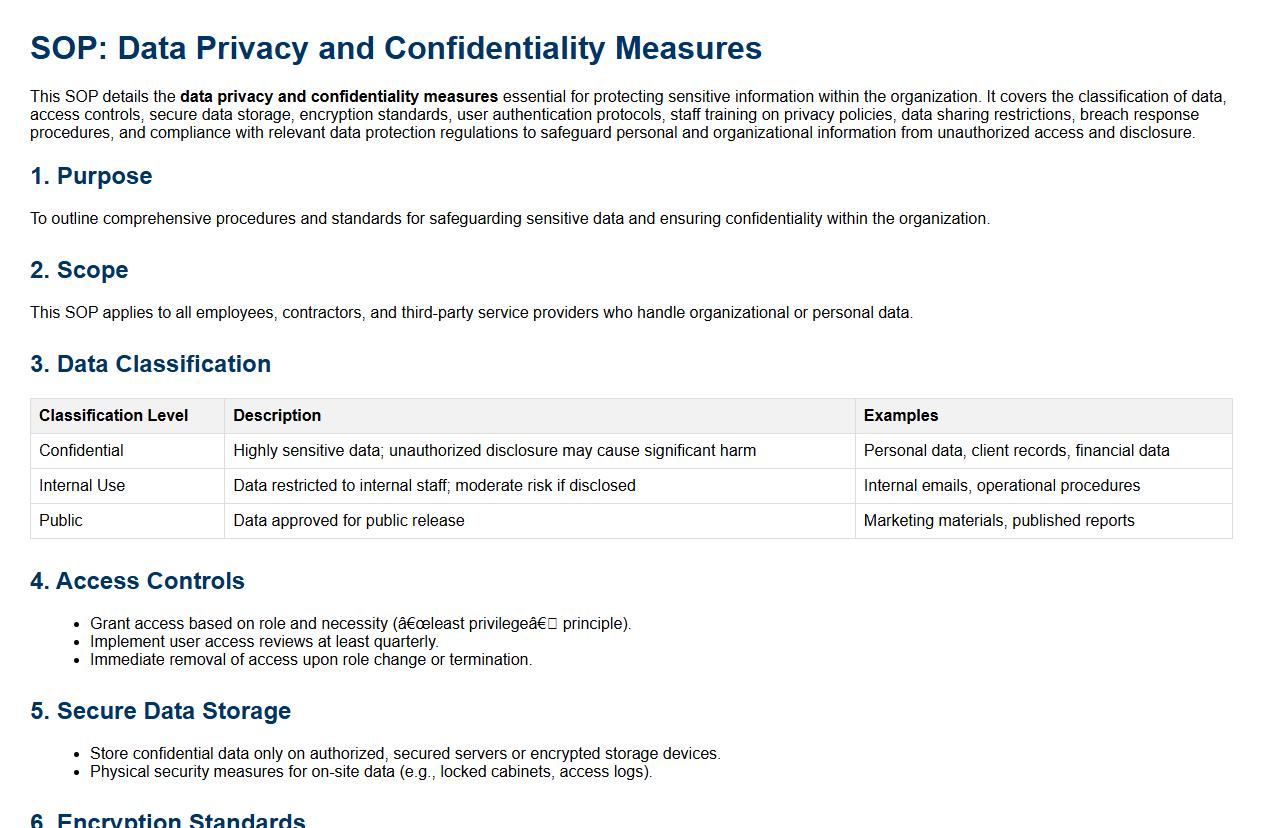
This SOP details the data privacy and confidentiality measures essential for protecting sensitive information within the organization. It covers the classification of data, access controls, secure data storage, encryption standards, user authentication protocols, staff training on privacy policies, data sharing restrictions, breach response procedures, and compliance with relevant data protection regulations to safeguard personal and organizational information from unauthorized access and disclosure.
Data review and audit trail documentation.

This SOP details the processes for data review and audit trail documentation, encompassing data verification, validation procedures, audit trail creation, and maintenance. It ensures accuracy, traceability, and compliance with regulatory standards by providing systematic guidelines for reviewing data entries, recording changes, and preserving audit trails to support transparency and accountability in data management.
Archival and secure deletion of obsolete data.

This SOP details the procedures for archival and secure deletion of obsolete data, including identification of data eligible for archiving or deletion, secure data transfer to archival storage, implementation of encryption and access controls, verification of data integrity post-transfer, schedules and policies for data retention, methods for secure deletion to prevent data recovery, compliance with legal and regulatory requirements, and documentation of all archival and deletion activities to ensure data privacy and organizational security.
Primary Steps for Accurate Data Entry and Validation
The SOP emphasizes accurate data entry through standardized templates and double-entry verification methods. It requires personnel to perform real-time validation checks to minimize errors. Additionally, the use of automated validation tools is mandated to ensure data integrity from the outset.
Protocols for Handling Data Discrepancies or Errors
The SOP specifies immediate documentation and reporting of data discrepancies upon detection. It mandates a formal review process involving data managers to correct and resolve issues efficiently. Root cause analysis is also required to prevent future recurrence of similar errors.
User Access Levels and Authorization for Data Management
The SOP defines multiple user access levels based on roles, ensuring only authorized personnel can modify or approve data. Role-based access control (RBAC) is implemented to enhance data security and accountability. Regular audits of access permissions are also outlined to maintain strict control.
Data Backup and Recovery Procedures
The SOP mandates daily data backup to secure, offsite locations with encrypted storage methods. It outlines systematic recovery processes to restore data quickly in case of loss or corruption. Regular testing of backup integrity and recovery protocols is also a critical component.
Documentation and Audit Trail Requirements for Data Changes
The SOP requires comprehensive audit trails capturing all data modifications, including user identity, timestamp, and reason for change. All documentation must be stored securely and remain accessible for compliance audits. This ensures transparency and accountability throughout the data lifecycle.
