
A SOP Template for Data Entry Procedures provides a standardized framework to ensure accuracy and consistency in data input tasks. This template outlines step-by-step instructions, quality checks, and validation processes to minimize errors and maintain data integrity. Utilizing a clear and comprehensive SOP enhances workflow efficiency and supports compliance with organizational standards.
Data collection source verification and authorization.

This SOP defines the procedures for data collection source verification and authorization, including the identification and validation of data sources, verification of data accuracy and reliability, authorization protocols for data access and collection, documentation of source credentials, and compliance with data privacy and security standards. The objective is to ensure the integrity and authenticity of collected data by implementing robust verification and approval processes.
Standardized data entry formats and field requirements.
This SOP establishes standardized data entry formats and field requirements to ensure consistency, accuracy, and efficiency in data collection and management processes. It defines specific data input formats, mandatory field criteria, validation rules, and error handling protocols. The objective is to minimize data entry errors, streamline data integration, and enhance overall data quality across all systems and applications.
Step-by-step data input procedures for each platform/system.
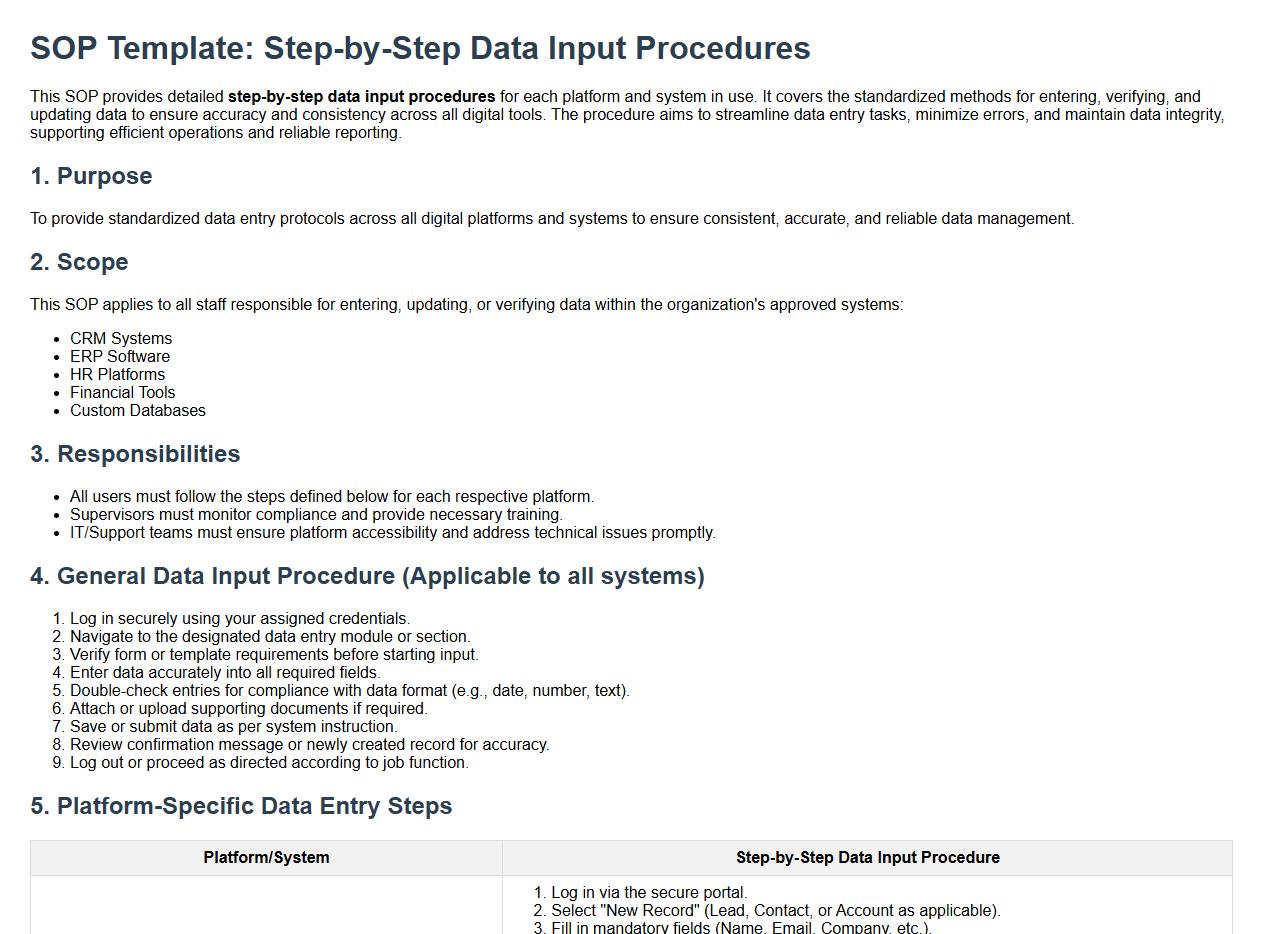
This SOP provides detailed step-by-step data input procedures for each platform and system in use. It covers the standardized methods for entering, verifying, and updating data to ensure accuracy and consistency across all digital tools. The procedure aims to streamline data entry tasks, minimize errors, and maintain data integrity, supporting efficient operations and reliable reporting.
Unique user login and access control protocols.
This SOP defines unique user login and access control protocols to enhance system security by ensuring that each user has a distinct login credential. It includes procedures for user authentication, password policies, role-based access controls, session management, and monitoring to prevent unauthorized access and protect sensitive information. The objective is to maintain the integrity and confidentiality of system resources through stringent access management practices.
Data quality checks and validation rules.
This SOP details the procedures for data quality checks and validation rules, including the identification of data accuracy, completeness, consistency, and reliability requirements. It covers methods for implementing validation rules, executing automated and manual data quality assessments, managing exceptions, and ensuring data integrity throughout the data lifecycle. The goal is to maintain high-quality data standards to support accurate reporting, analysis, and decision-making processes.
Handling of duplicate, missing, or inconsistent entries.

This SOP details the procedures for handling duplicate, missing, or inconsistent entries in data management systems. It covers identification techniques, verification processes, correction protocols, and prevention measures to ensure data integrity and accuracy. The goal is to maintain reliable and consistent data by addressing errors promptly and effectively.
Secure data storage, backup, and recovery guidelines.

This SOP provides comprehensive secure data storage, backup, and recovery guidelines to ensure the protection, integrity, and availability of organizational data. It covers best practices for data encryption, access controls, regular backup schedules, offsite backup storage, and procedures for data recovery in case of data loss or corruption. The goal is to minimize risks associated with data breaches, hardware failures, and other incidents while maintaining compliance with relevant data protection regulations.
Error identification, correction, and escalation process.

This SOP details the error identification, correction, and escalation process, encompassing the systematic detection of errors, immediate rectification methods, documentation of issues, communication protocols for escalating unresolved problems, and continuous monitoring to prevent recurrence. The objective is to ensure efficient handling of errors to maintain quality, minimize disruptions, and facilitate timely resolution through appropriate channels.
Scheduled review, audit, and update of entered data.

This SOP defines the process for the scheduled review, audit, and update of entered data, ensuring data accuracy, consistency, and integrity. It covers routine verification of data entries, identification and correction of discrepancies, documentation of audit findings, and timely updates to maintain reliable and up-to-date information. The procedure supports data quality management and compliance with organizational standards.
Confidentiality, privacy, and data protection compliance measures.

This SOP details confidentiality, privacy, and data protection compliance measures, encompassing guidelines for handling sensitive information, ensuring data privacy, managing access controls, implementing data encryption, securing storage and transmission of data, conducting regular audits and training, and adhering to relevant legal and regulatory requirements. The objective is to protect personal and organizational data from unauthorized access, breaches, and misuse while maintaining trust and compliance with applicable data protection laws.
What are the required data fields and formats specified in the SOP for Data Entry Procedures?
The SOP mandates that all data entries must include mandatory fields such as name, date, identification number, and contact details. Each field must follow strict format guidelines, for example, dates must be in YYYY-MM-DD format and IDs should be alphanumeric with no special characters. Adhering to these specifications ensures consistency and accuracy throughout the data entry process.
Which validation checks must be performed before submitting any data entry?
The SOP requires a series of validation checks including verifying field completeness, correct format adherence, and cross-referencing data with source documents. Additionally, error detection protocols like duplicate checks and logical consistency tests must be conducted. These validation steps are crucial to prevent data inaccuracies and maintain database integrity.
What steps must be followed for correcting or updating existing data entries according to the SOP?
To correct or update data, the SOP instructs users to first log the requested change with reason and date, then submit the update request through the designated approval workflow. Once approved, the changes must be applied promptly, ensuring the previous information is archived for audit purposes. This controlled process maintains transparency and accountability in data management.
Who is authorized to perform, verify, and approve data entries within the SOP's guidelines?
The SOP strictly defines specific roles; only trained data entry operators are authorized to input data, while supervisors or quality assurance personnel verify and approve the entries. Authorization levels are assigned based on expertise and responsibility, preventing unauthorized data manipulation. This hierarchical control is essential for upholding data quality standards.
What process is outlined in the SOP for reporting and documenting data entry errors or discrepancies?
The SOP outlines a formal error reporting process requiring immediate documentation of discrepancies in a standardized error log. The responsible personnel must notify supervisors and initiate corrective actions as per the escalation protocol detailed in the SOP. Proper documentation ensures traceability and helps in continuous improvement of data handling practices.
