
A SOP Template for Laboratory Sample Handling ensures standardized procedures for the collection, labeling, storage, and transportation of samples to maintain integrity and prevent contamination. This template provides clear, step-by-step instructions to improve accuracy, traceability, and compliance with regulatory requirements. Proper use of the SOP Template for Laboratory Sample Handling enhances consistency and reliability in laboratory testing outcomes.
Sample receipt and accessioning procedures.
This SOP details the sample receipt and accessioning procedures to ensure accurate logging, identification, and tracking of all incoming samples. It covers steps for verifying sample integrity, labeling, assigning unique accession numbers, recording sample details in the laboratory information system, and proper storage before analysis. The goal is to maintain sample traceability, prevent mix-ups, and uphold the quality and reliability of laboratory testing processes.
Sample labeling and identification standards.

This SOP establishes sample labeling and identification standards to ensure accurate, consistent, and reliable tracking of samples throughout the workflow. It covers protocols for label content, format, placement, and handling to prevent misidentification and contamination. This ensures traceability, data integrity, and compliance with regulatory and quality management requirements, facilitating effective sample management and analysis.
Chain of custody documentation and tracking.
This SOP details the process of chain of custody documentation and tracking, ensuring the accurate and secure handling, transfer, and documentation of evidence or samples from collection to final disposition. It includes procedures for labeling, recording, verifying transfers, maintaining integrity, and preventing contamination or tampering, thereby supporting accountability and traceability throughout the entire custody lifecycle.
Sample storage conditions and organization.
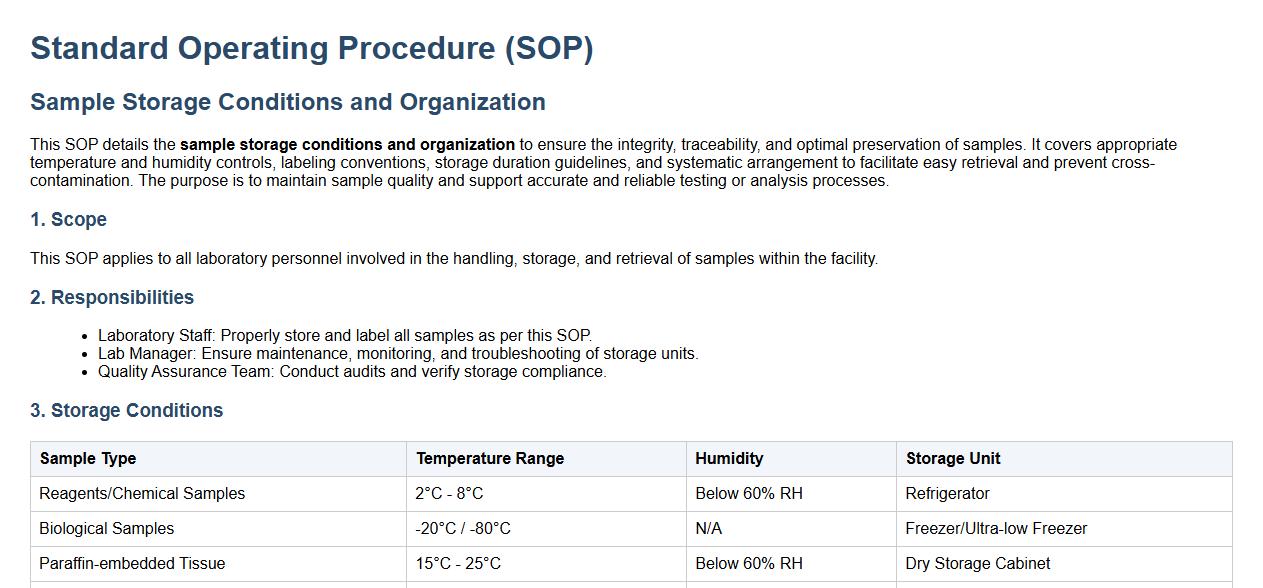
This SOP details the sample storage conditions and organization to ensure the integrity, traceability, and optimal preservation of samples. It covers appropriate temperature and humidity controls, labeling conventions, storage duration guidelines, and systematic arrangement to facilitate easy retrieval and prevent cross-contamination. The purpose is to maintain sample quality and support accurate and reliable testing or analysis processes.
Sample preparation and processing guidelines.
This SOP defines the standardized sample preparation and processing guidelines, covering steps such as sample collection, labeling, storage, and handling procedures. It includes protocols for proper equipment usage, contamination prevention, processing timelines, and quality control measures to ensure accurate and reliable analytical results. The objective is to maintain sample integrity throughout the preparation and processing stages, facilitating consistent and reproducible outcomes in laboratory testing.
Decontamination and contamination prevention measures.

This SOP details decontamination and contamination prevention measures essential for maintaining a safe and hygienic environment. It covers protocols for cleaning and disinfecting equipment, surfaces, and personnel; procedures to prevent cross-contamination; guidelines for handling hazardous materials; and the use of personal protective equipment (PPE). The purpose is to minimize contamination risks, ensure compliance with health standards, and protect both personnel and the integrity of the work processes.
Handling of hazardous or infectious materials.

This SOP provides comprehensive guidelines for the handling of hazardous or infectious materials, including proper identification, safe storage, usage protocols, personal protective equipment requirements, spill response procedures, disposal methods, and employee training. The aim is to minimize health risks, prevent contamination, and ensure compliance with regulatory standards for safe management of dangerous substances in the workplace.
Sample retention and disposal procedures.

This SOP details the sample retention and disposal procedures, covering the proper handling, storage, documentation, and disposal of samples to ensure compliance with regulatory requirements and maintain data integrity. It includes guidelines for sample labeling, retention periods, storage conditions, chain of custody, criteria for sample disposal, and environmentally responsible disposal methods. The purpose is to safeguard sample quality, prevent contamination, and ensure timely and secure disposal of obsolete or expired samples.
Quality assurance and control checks.

This SOP details the quality assurance and control checks procedures, covering systematic inspection methods, standards compliance, material verification, process monitoring, defect identification, corrective actions, documentation protocols, and continuous improvement strategies to ensure product consistency, reliability, and customer satisfaction.
Incident and deviation reporting protocols.

This SOP details incident and deviation reporting protocols to ensure timely and accurate documentation of any irregularities, accidents, or non-conformities within operational processes. It covers the identification, reporting, investigation, and corrective actions to maintain compliance, improve safety, and enhance overall organizational performance.
What are the key steps outlined for receiving and logging laboratory samples according to the SOP?
Receiving laboratory samples involves verifying the sample's condition upon arrival and ensuring it matches the accompanying documentation. Samples must be logged into the laboratory information system with accurate details including time of receipt and sample type. This process ensures traceability and proper management of all laboratory specimens.
Which labeling requirements must be followed for all laboratory samples as specified in the SOP?
All laboratory samples must have clear and durable labels that include patient identification, sample type, collection date, and time. Labels should be securely affixed to prevent detachment during handling and processing. Accurate labeling is critical to avoid mix-ups and maintain sample integrity.
How does the SOP define proper storage conditions for different sample types?
The SOP mandates that samples be stored under specific temperature and environmental conditions based on their type to preserve their integrity. For example, blood samples may require refrigeration while others need freezing or ambient storage. Proper storage prevents degradation and ensures reliable test results.
What are the procedures described for handling and preventing sample contamination in the SOP?
The SOP emphasizes using sterile techniques and protective equipment to prevent contamination during sample handling. This includes wearing gloves, using clean tools, and avoiding cross-contact between specimens. Following these procedures preserves sample quality and ensures accurate laboratory analyses.
According to the SOP, what documentation is required during the disposal or return of laboratory samples?
Documentation must include a disposal or return log specifying sample identification, disposal date, method, and personnel responsible. This record ensures accountability and compliance with regulatory and safety standards. Proper documentation supports traceability and audit readiness.
