
A SOP Template for Surgical Instrument Handling ensures standardized procedures for the proper cleaning, sterilization, and storage of surgical tools. This template helps maintain instrument integrity and patient safety by outlining step-by-step protocols for healthcare staff. Implementing these guidelines reduces contamination risks and promotes compliance with healthcare regulations.
Instrument receipt and verification protocol.

This SOP establishes the instrument receipt and verification protocol to ensure accurate and efficient handling of incoming instruments. It details procedures for inspecting shipment condition, verifying instrument specifications against purchase orders, documenting receipt with proper records, and reporting discrepancies or damages. The goal is to maintain quality control, prevent operational delays, and ensure the integrity of instruments before they are integrated into workflows or inventories.
Pre-sterilization cleaning procedures.
This SOP details the pre-sterilization cleaning procedures necessary to ensure medical instruments and equipment are free from contaminants before sterilization. It covers steps for manual and automated cleaning, selection and use of cleaning agents, inspection of items for residue or damage, and proper handling to maintain aseptic conditions. Adhering to these procedures minimizes infection risks and improves the effectiveness of subsequent sterilization processes.
Inspection and quality check for instrument integrity.

This SOP details the process for inspection and quality check for instrument integrity, encompassing routine visual inspections, functional testing, calibration verification, identification of wear and damage, documentation of findings, and corrective actions. The objective is to ensure that all instruments meet specified standards for accuracy, reliability, and safety, thereby maintaining optimal performance and compliance with regulatory requirements.
Proper loading and arrangement of instruments for sterilization.

This SOP describes the proper loading and arrangement of instruments for sterilization to ensure effective sterilization, prevent damage, and maintain instrument integrity. It includes guidelines for cleaning instruments prior to loading, correct placement and spacing within sterilization trays, selection of appropriate sterilization packaging materials, and proper arrangement to allow steam or sterilizing agent penetration. The procedure aims to maximize sterilization efficacy, reduce the risk of contamination, and promote patient safety in healthcare settings.
Documentation and labeling of instrument sets.

This SOP details the documentation and labeling of instrument sets, encompassing the proper identification, organization, and tracking of surgical instruments. It ensures accurate recording of instrument contents, sterilization status, and procedural usage to maintain safety, efficiency, and compliance with healthcare standards. This process supports effective inventory management and prevents errors during surgical procedures.
Sterilization methods and cycle parameters.
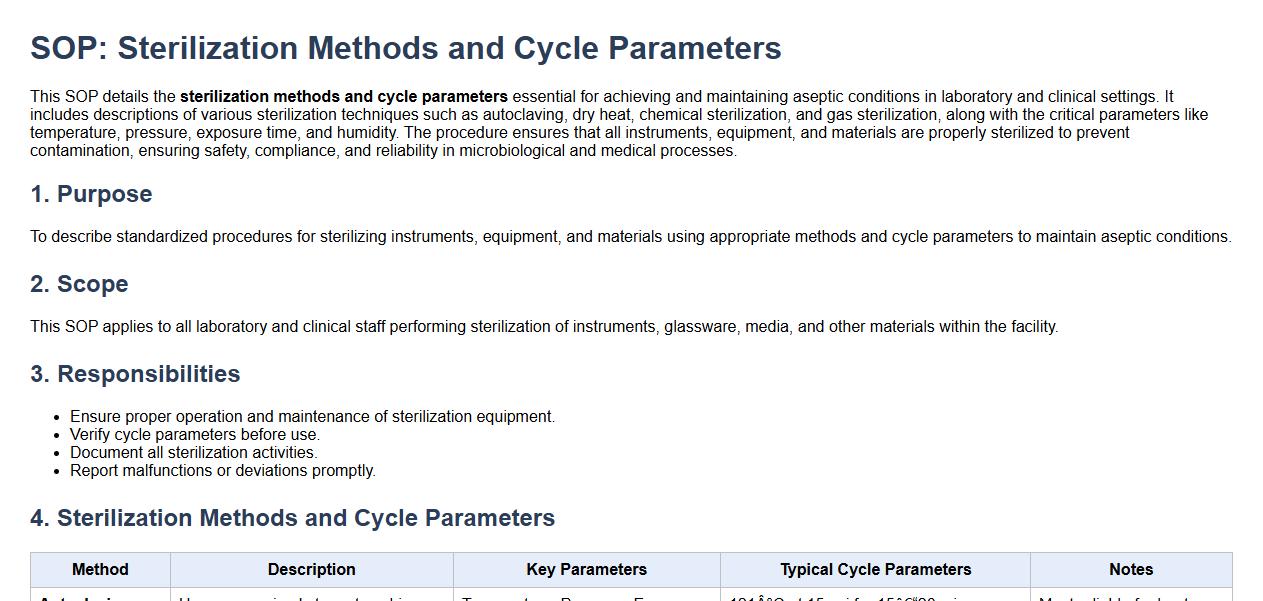
This SOP details the sterilization methods and cycle parameters essential for achieving and maintaining aseptic conditions in laboratory and clinical settings. It includes descriptions of various sterilization techniques such as autoclaving, dry heat, chemical sterilization, and gas sterilization, along with the critical parameters like temperature, pressure, exposure time, and humidity. The procedure ensures that all instruments, equipment, and materials are properly sterilized to prevent contamination, ensuring safety, compliance, and reliability in microbiological and medical processes.
Post-sterilization instrument inspection and validation.

This SOP details the process for post-sterilization instrument inspection and validation, including procedures for visually inspecting surgical instruments for cleanliness, verifying sterilization indicators, conducting functional tests, and documenting inspection results. The aim is to ensure all instruments are sterile, safe, and ready for use in medical procedures, thereby preventing infections and maintaining patient safety.
Instrument storage and inventory management.

This SOP details instrument storage and inventory management, covering proper labeling, categorization, and secure storage of instruments, routine inventory checks, maintenance schedules, documentation protocols, and procedures for issuing and returning equipment. The goal is to ensure accurate tracking, prevent loss or damage, and maintain operational efficiency through standardized management of all instruments.
Preparation and transfer of instruments to the surgical field.

This SOP details the preparation and transfer of instruments to the surgical field, covering the sterilization process, assembly and inspection of surgical instruments, proper handling techniques to maintain sterility, and the systematic transfer of instruments to the sterile field during surgery. The objective is to ensure aseptic conditions, minimize infection risks, and support efficient surgical procedures by maintaining instrument integrity and readiness.
Instrument collection, decontamination, and waste disposal procedures.

This SOP details the procedures for instrument collection, decontamination, and waste disposal, ensuring the safe and effective handling of medical or laboratory instruments. It covers proper collection techniques, thorough cleaning and sterilization processes to prevent contamination, and the correct disposal methods for hazardous and non-hazardous waste. Adhering to these procedures is essential for maintaining hygiene, preventing infection, and complying with regulatory standards.
Key Procedures for Sterile Handling of Surgical Instruments
The SOP mandates strict adherence to aseptic techniques during the handling of surgical instruments to prevent contamination. Instruments must be handled with sterile gloves and transferred only within sterile fields. Proper storage and immediate sterilization post-use are also critical to maintain sterility.
Criteria for Accepting and Rejecting Surgical Instruments Before Use
The SOP requires a thorough visual inspection of instruments for cleanliness, integrity, and functionality before use. Any instrument showing signs of damage, corrosion, or residue is strictly rejected. Only instruments meeting all quality criteria are accepted for surgical procedures.
Documentation and Traceability Requirements for Instrument Handling
The SOP specifies comprehensive documentation including batch numbers, sterilization dates, and handling logs to ensure full traceability. Each instrument set must be tracked from sterilization to use and back to reprocessing. Accurate records facilitate audits and maintain high safety standards.
Decontamination Steps for Post-Operative Instrument Processing
Post-operative handling requires immediate removal of gross debris followed by automated or manual cleaning with enzymatic detergents. The SOP emphasizes thorough rinsing and drying before sterilization. This multi-step decontamination reduces microbial load and prepares instruments for safe reuse.
PPE Mandated During the Handling of Surgical Instruments
The SOP mandates the use of gloves, masks, gowns, and eye protection to safeguard personnel during instrument handling. PPE requirements vary slightly depending on the stage of handling but always prioritize user and patient safety. Proper donning and doffing procedures are also outlined to minimize contamination risks.
