
A SOP Template for Incident Reporting in Healthcare ensures a standardized process for documenting and managing adverse events, enhancing patient safety and regulatory compliance. It outlines clear steps for identifying, reporting, and analyzing incidents to prevent future occurrences. This template promotes accountability and timely communication among healthcare professionals.
Incident identification and initial response procedures.
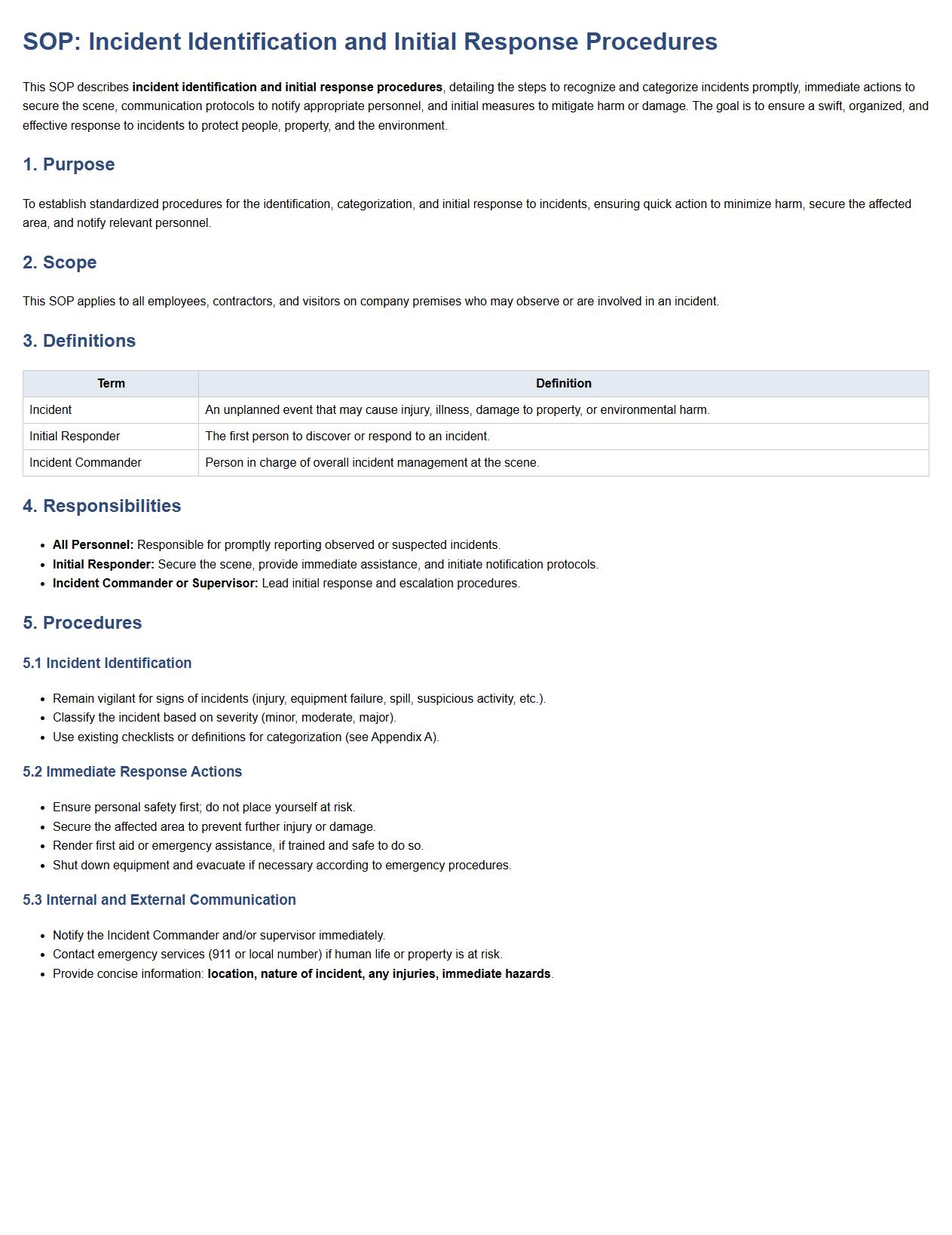
This SOP describes incident identification and initial response procedures, detailing the steps to recognize and categorize incidents promptly, immediate actions to secure the scene, communication protocols to notify appropriate personnel, and initial measures to mitigate harm or damage. The goal is to ensure a swift, organized, and effective response to incidents to protect people, property, and the environment.
Immediate notification of relevant personnel and authorities.

This SOP describes the process for immediate notification of relevant personnel and authorities to ensure timely communication during emergencies or critical incidents. It outlines the steps to quickly identify key contacts, use appropriate communication channels, and provide accurate information to enable a coordinated response and minimize potential risks or damages. The goal is to maintain clear and efficient communication pathways to safeguard personnel, property, and operations.
Documentation requirements for incident details and evidence collection.

This SOP defines the documentation requirements for incident details and evidence collection, ensuring accurate, thorough, and timely recording of all relevant information related to incidents. It covers the proper methods for capturing evidence, maintaining chain of custody, completing incident reports, and storing documentation securely to support investigations, compliance, and future safety improvements.
Guidelines for patient safety and containment of incident impact.
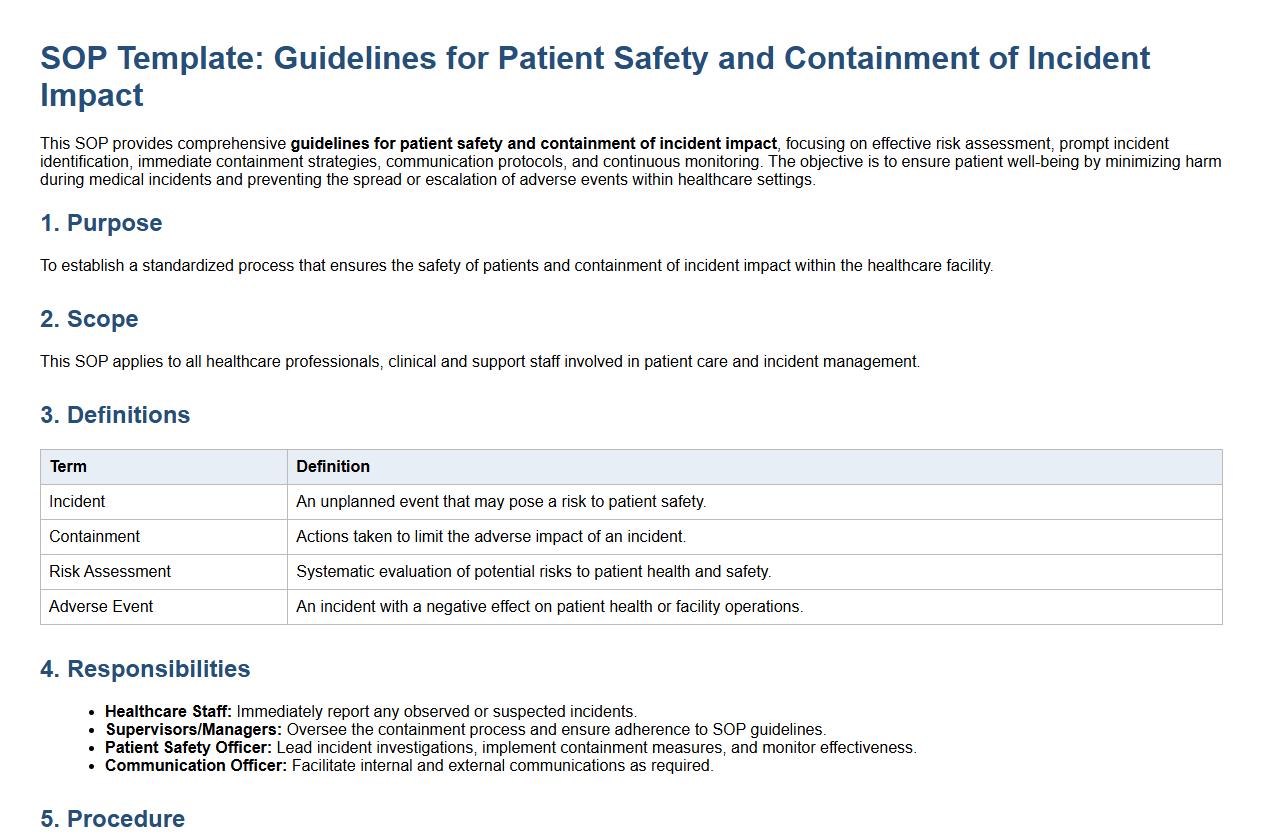
This SOP provides comprehensive guidelines for patient safety and containment of incident impact, focusing on effective risk assessment, prompt incident identification, immediate containment strategies, communication protocols, and continuous monitoring. The objective is to ensure patient well-being by minimizing harm during medical incidents and preventing the spread or escalation of adverse events within healthcare settings.
Step-by-step incident reporting form completion.

This SOP provides detailed instructions for the step-by-step incident reporting form completion, ensuring accurate, timely, and thorough documentation of workplace incidents. It covers identifying the type of incident, collecting essential details such as date, time, location, and personnel involved, describing the incident clearly and objectively, noting immediate actions taken, and recommending preventive measures. Proper completion of the incident reporting form facilitates effective incident management, investigation, and compliance with organizational and regulatory requirements.
Escalation process to management and risk teams.

This SOP defines the escalation process to management and risk teams, detailing the steps for timely and effective communication of issues, identification of risk levels, roles and responsibilities of involved personnel, criteria for escalation, documentation requirements, and follow-up procedures. The goal is to ensure that potential risks and critical incidents are promptly addressed by appropriate management and risk teams to mitigate impact and support informed decision-making.
Timelines and channels for incident reporting submission.
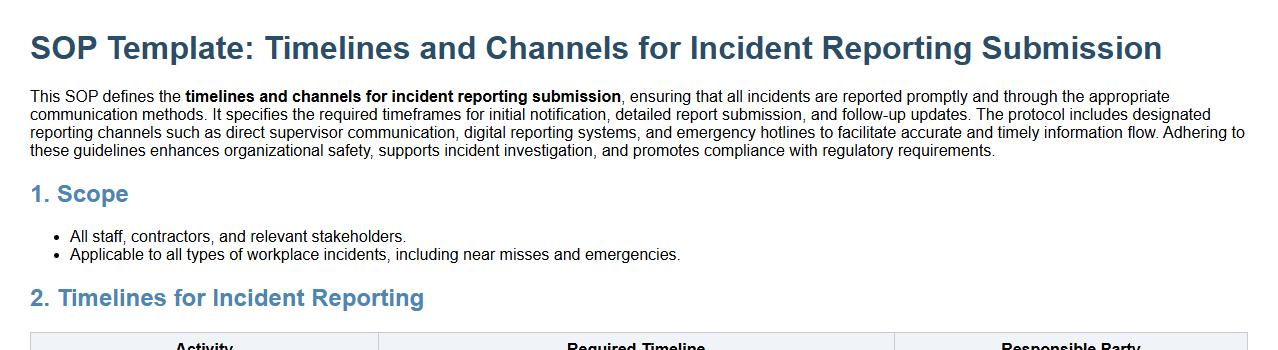
This SOP defines the timelines and channels for incident reporting submission, ensuring that all incidents are reported promptly and through the appropriate communication methods. It specifies the required timeframes for initial notification, detailed report submission, and follow-up updates. The protocol includes designated reporting channels such as direct supervisor communication, digital reporting systems, and emergency hotlines to facilitate accurate and timely information flow. Adhering to these guidelines enhances organizational safety, supports incident investigation, and promotes compliance with regulatory requirements.
Procedures for root cause analysis initiation.
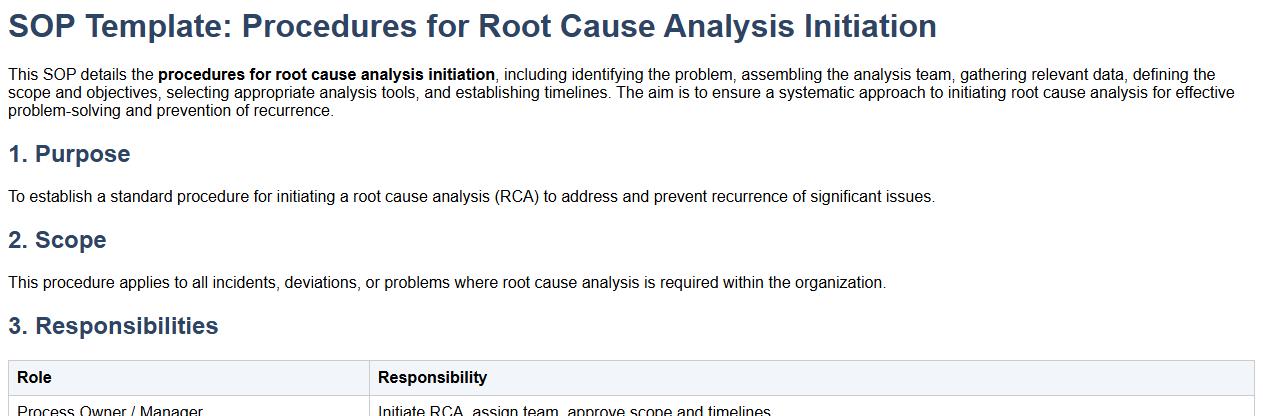
This SOP details the procedures for root cause analysis initiation, including identifying the problem, assembling the analysis team, gathering relevant data, defining the scope and objectives, selecting appropriate analysis tools, and establishing timelines. The aim is to ensure a systematic approach to initiating root cause analysis for effective problem-solving and prevention of recurrence.
Follow-up actions, corrective measures, and preventive steps.
This SOP details the process for follow-up actions, corrective measures, and preventive steps to address identified issues or non-conformities. It includes procedures for investigating root causes, implementing timely corrective actions, monitoring their effectiveness, and establishing preventive measures to avoid recurrence. The objective is to ensure continuous improvement and compliance with quality and safety standards through systematic problem resolution and risk mitigation.
Confidentiality, data protection, and documentation retention protocols.

This SOP establishes guidelines for confidentiality, data protection, and documentation retention protocols, ensuring the secure handling of sensitive information, compliance with data privacy regulations, and systematic retention and disposal of documents. It covers procedures for safeguarding personal and organizational data, access controls, encryption standards, data breach response, and retention schedules to maintain legal and operational integrity.
What key steps must be followed when documenting an incident according to the SOP for Incident Reporting in Healthcare?
The incident documentation process requires a detailed and accurate recording of the event. This includes identifying the incident, describing what occurred, and noting the immediate actions taken. All relevant facts must be gathered promptly to ensure completeness and clarity.
Which types of incidents are required to be reported as per the SOP guidelines?
The SOP mandates reporting of all adverse events, near misses, and unsafe conditions that may impact patient safety. This includes clinical errors, equipment malfunctions, and violations of safety protocols. Comprehensive reporting ensures proactive risk management in healthcare settings.
Who is responsible for submitting and reviewing incident reports under the SOP?
The healthcare staff directly involved in the incident must submit the initial report. Subsequently, designated supervisors or risk management personnel review and evaluate each incident report. This dual responsibility guarantees accountability and thorough analysis.
What is the specified timeline for reporting an incident as outlined in the SOP?
The SOP requires incident reports to be submitted immediately or within 24 hours of occurrence. Timely reporting facilitates swift response and mitigation of potential harm. Delayed reports may compromise the effectiveness of corrective actions.
How does the SOP ensure confidentiality and data protection in the incident reporting process?
The SOP emphasizes strict confidentiality protocols and secure data handling measures to protect patient and staff information. Access to incident reports is limited to authorized personnel only. Compliance with data protection laws is mandatory throughout the reporting process.
