
A SOP Template for Finished Goods Inspection in Manufacturing outlines standardized procedures to ensure product quality and compliance before shipment. It includes detailed inspection criteria, documentation requirements, and corrective actions to address defects. This template helps maintain consistency, reduce errors, and improve overall manufacturing efficiency.
Receiving finished goods from production for inspection.

This SOP details the process for receiving finished goods from production for inspection, including verifying the quantity and quality of products delivered, cross-checking production documentation, identifying and segregating defective items, recording inspection results, and coordinating with production and quality control teams to ensure compliance with quality standards before goods proceed to storage or dispatch.
Verification of product quantity against production order.

This SOP details the process for verification of product quantity against production order, ensuring accurate matching of manufactured product quantities with specified production requirements. It includes steps for measuring, recording, and validating product counts, cross-referencing production orders, and addressing discrepancies to maintain quality control and production efficiency.
Visual inspection for defects, damages, or contamination.

This SOP details the procedure for performing a visual inspection for defects, damages, or contamination, ensuring that all products and materials are thoroughly examined before processing or distribution. It outlines the criteria for identifying common defects, damage types, and contamination indicators, as well as the steps for documenting findings and initiating corrective actions. The purpose of this SOP is to maintain high-quality standards, prevent product recalls, and ensure customer safety by promptly detecting and addressing any quality issues.
Measurement and testing of critical dimensions and specifications.

This SOP details the standardized process for measurement and testing of critical dimensions and specifications, ensuring accuracy and consistency in product quality. It covers the selection and calibration of measuring instruments, step-by-step measurement procedures, data recording methodologies, and criteria for acceptance or rejection based on specified tolerances. Adhering to this SOP guarantees precise verification of dimensional requirements, reduces variability, and supports compliance with industry standards and customer specifications.
Functional testing to ensure operational performance.
This SOP describes the process for functional testing to ensure operational performance, detailing the steps to verify that systems, components, or software operate according to specified requirements. It includes test planning, execution of test cases, result documentation, identification and resolution of defects, and validation of performance metrics to guarantee reliability and efficiency in real-world conditions.
Documentation review for batch records and traceability.

This SOP details the process for documentation review for batch records and traceability, ensuring all manufacturing batch records are accurately completed, reviewed, and verified for compliance with regulatory standards. It covers procedures for cross-checking data entries, verifying traceability of materials and components used, identifying discrepancies, and maintaining thorough documentation to support product quality and safety throughout production. The aim is to guarantee the integrity and traceability of batch information, facilitating effective quality control and regulatory audits.
Identification and segregation of non-conforming products.

This SOP details the process for the identification and segregation of non-conforming products, ensuring that products not meeting quality standards are promptly recognized and separated from conforming goods. It covers procedures for inspection, labeling, documentation, and storage of non-conforming items, facilitating effective quality control and preventing the mixing of defective products with acceptable inventory. This protocol aims to maintain product integrity, enhance customer satisfaction, and comply with regulatory requirements.
Completion of finished goods inspection checklist and report.

This SOP details the process for the completion of finished goods inspection checklist and report, including inspection criteria, documentation procedures, quality assessment, adherence to product specifications, identification of defects, corrective action recommendations, and final approval. The objective is to ensure that all finished products meet quality standards before shipment, guaranteeing customer satisfaction and compliance with regulatory requirements.
Labelling and approval of inspected goods for release to storage or dispatch.

This SOP details the labelling and approval process for inspected goods prior to their release to storage or dispatch. It covers the verification of inspection criteria, accurate labelling requirements, documentation procedures, and the authorization steps needed to ensure that only compliant and approved goods proceed to the next stage, maintaining quality control and traceability throughout the supply chain.
Escalation procedure for handling major defects or repeated non-conformance.
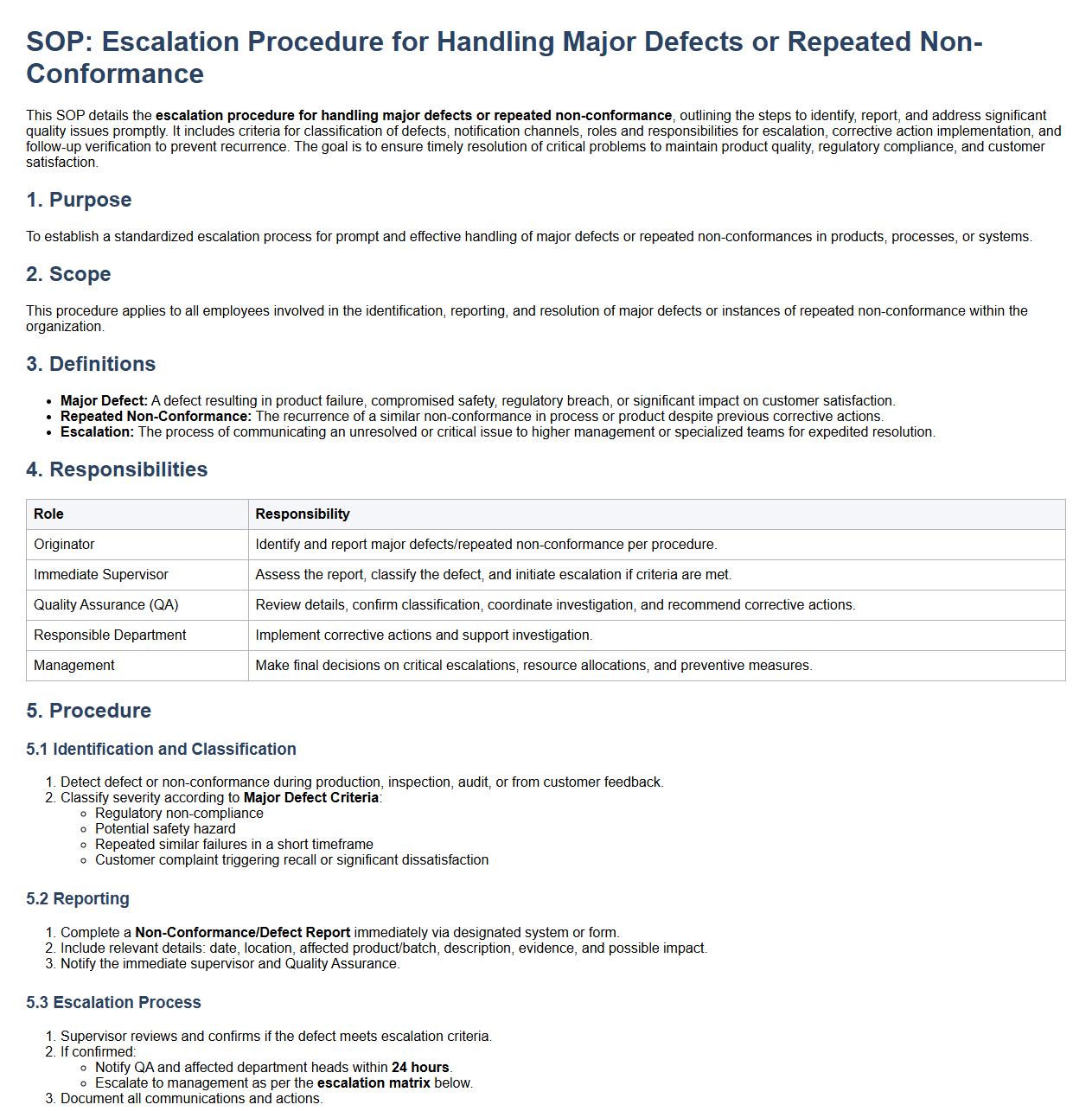
This SOP details the escalation procedure for handling major defects or repeated non-conformance, outlining the steps to identify, report, and address significant quality issues promptly. It includes criteria for classification of defects, notification channels, roles and responsibilities for escalation, corrective action implementation, and follow-up verification to prevent recurrence. The goal is to ensure timely resolution of critical problems to maintain product quality, regulatory compliance, and customer satisfaction.
Key Criteria for Accepting or Rejecting Finished Goods
The SOP specifies strict quality standards that finished goods must meet to be accepted. It highlights criteria such as dimensional accuracy, aesthetic compliance, and functional performance. Any deviation from these standards leads to immediate rejection of the goods.
Specific Inspection Methods and Tools Mandated
The SOP mandates the use of calibrated measuring instruments and visual inspection techniques. Tools such as micrometers, gauges, and optical comparators are required for precise assessment. These methods ensure consistency and reliability in finished goods evaluation.
Documentation and Recordkeeping Requirements
The SOP enforces meticulous recordkeeping of inspection results, including dates, inspector names, and outcomes. All records must be securely stored and easily accessible for audits or traceability. This ensures transparency and accountability in the inspection process.
Handling and Reporting Non-Conforming Finished Goods
Non-conforming goods must be clearly identified and segregated as per the SOP guidelines. The SOP requires immediate reporting to the quality control manager and proper documentation of the issue. Corrective actions must be initiated promptly to prevent recurrence.
Authorization and Responsibility for Final Approval
The SOP assigns final approval authority to a designated quality assurance manager or qualified personnel. This individual reviews all inspection documentation before granting acceptance. This ensures that only products meeting all criteria enter the market.
