
The SOP Template for Manufacturing Order Processing streamlines the workflow by clearly defining each step from order receipt to production completion. It ensures consistency, accuracy, and compliance with industry standards while minimizing errors and delays. This template facilitates effective communication between departments, improving overall manufacturing efficiency.
Order receipt and verification procedures.

This SOP details the order receipt and verification procedures, encompassing the systematic process for receiving incoming orders, verifying order accuracy against purchase requests or contracts, checking quantities and specifications, documenting receipt details, and addressing discrepancies. The procedure ensures that all orders are accurately recorded and verified to maintain inventory integrity and support efficient supply chain operations.
Customer information validation and order confirmation steps.

This SOP details the customer information validation and order confirmation steps, encompassing the verification of customer details such as contact information and shipping addresses, ensuring data accuracy and completeness before processing orders. It also includes procedures for confirming order receipt with customers, validating payment methods, and verifying product availability to prevent errors and enhance customer satisfaction. The objective is to streamline the order management process, reduce errors, and provide a reliable confirmation system that fosters trust and clarity between the business and its customers.
Bill of materials preparation and approval protocols.
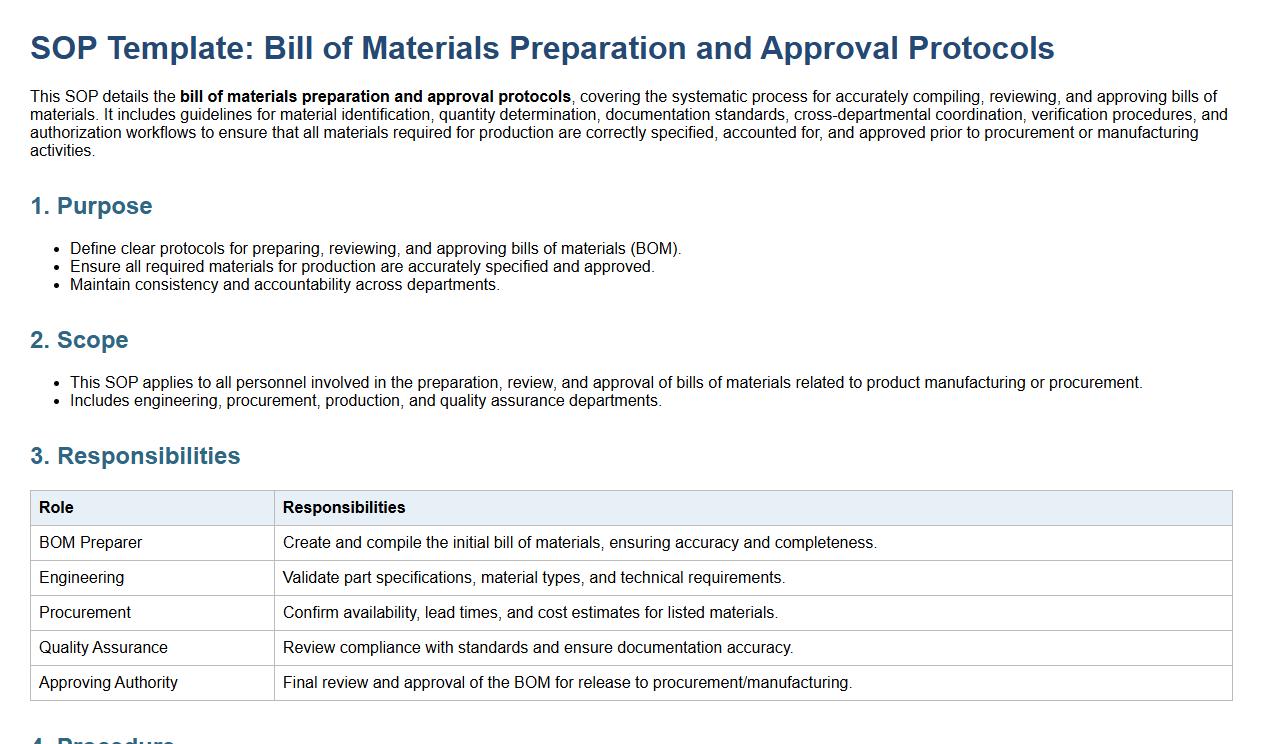
This SOP details the bill of materials preparation and approval protocols, covering the systematic process for accurately compiling, reviewing, and approving bills of materials. It includes guidelines for material identification, quantity determination, documentation standards, cross-departmental coordination, verification procedures, and authorization workflows to ensure that all materials required for production are correctly specified, accounted for, and approved prior to procurement or manufacturing activities.
Production scheduling and capacity planning.

This SOP details the processes for production scheduling and capacity planning, including forecasting demand, allocating resources, setting production timelines, balancing workload across facilities, optimizing equipment usage, and monitoring production progress. The goal is to ensure efficient use of production capacity, meet delivery deadlines, and adapt to changes in demand while minimizing downtime and costs.
Raw material requisition and inventory check process.

This SOP defines the raw material requisition and inventory check process, detailing steps for accurately requesting raw materials, verifying current inventory levels, ensuring timely procurement, and maintaining updated inventory records. The aim is to optimize inventory management, prevent material shortages, and support seamless production operations through consistent monitoring and control of raw material stocks.
Work order generation and job assignment procedures.
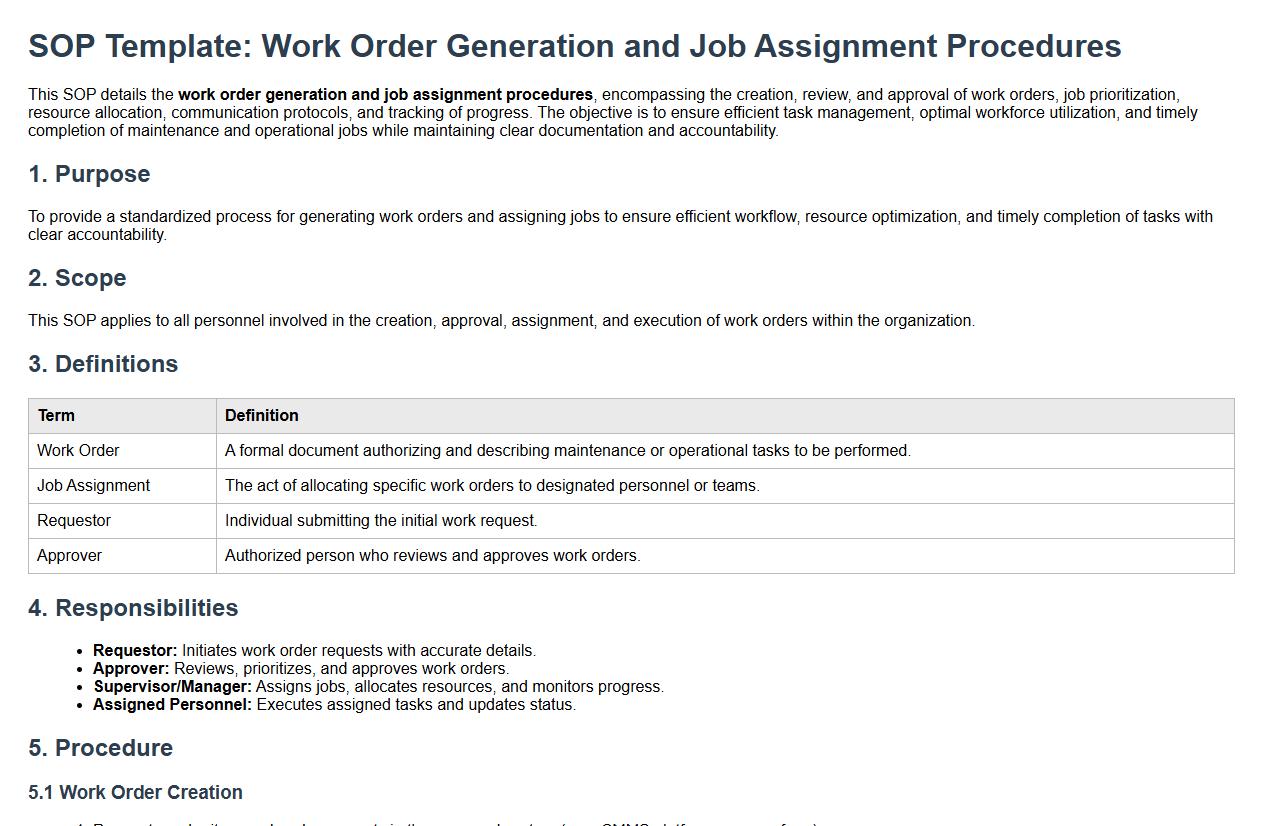
This SOP details the work order generation and job assignment procedures, encompassing the creation, review, and approval of work orders, job prioritization, resource allocation, communication protocols, and tracking of progress. The objective is to ensure efficient task management, optimal workforce utilization, and timely completion of maintenance and operational jobs while maintaining clear documentation and accountability.
In-process quality control and inspection checkpoints.
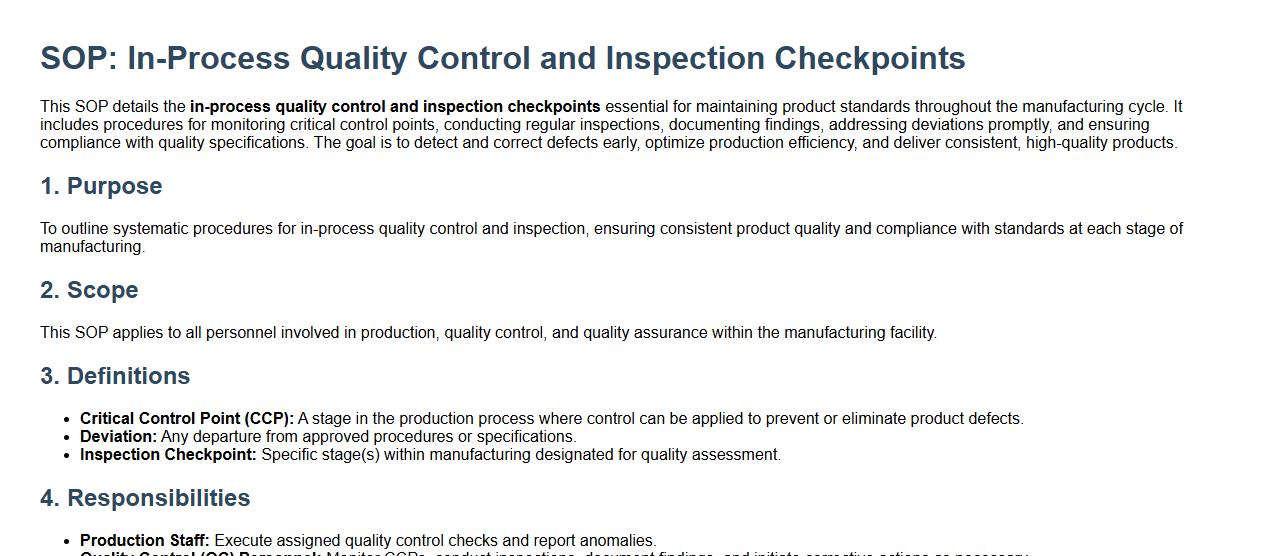
This SOP details the in-process quality control and inspection checkpoints essential for maintaining product standards throughout the manufacturing cycle. It includes procedures for monitoring critical control points, conducting regular inspections, documenting findings, addressing deviations promptly, and ensuring compliance with quality specifications. The goal is to detect and correct defects early, optimize production efficiency, and deliver consistent, high-quality products.
Finished goods packaging and labeling standards.

This SOP defines the finished goods packaging and labeling standards, covering packaging material requirements, labeling accuracy, compliance with regulatory guidelines, quality control procedures, packaging integrity checks, proper documentation, and shipment preparation. The goal is to ensure products are securely packaged and clearly labeled to maintain product quality and provide essential information to customers and regulatory authorities.
Order dispatch, shipping, and delivery documentation.
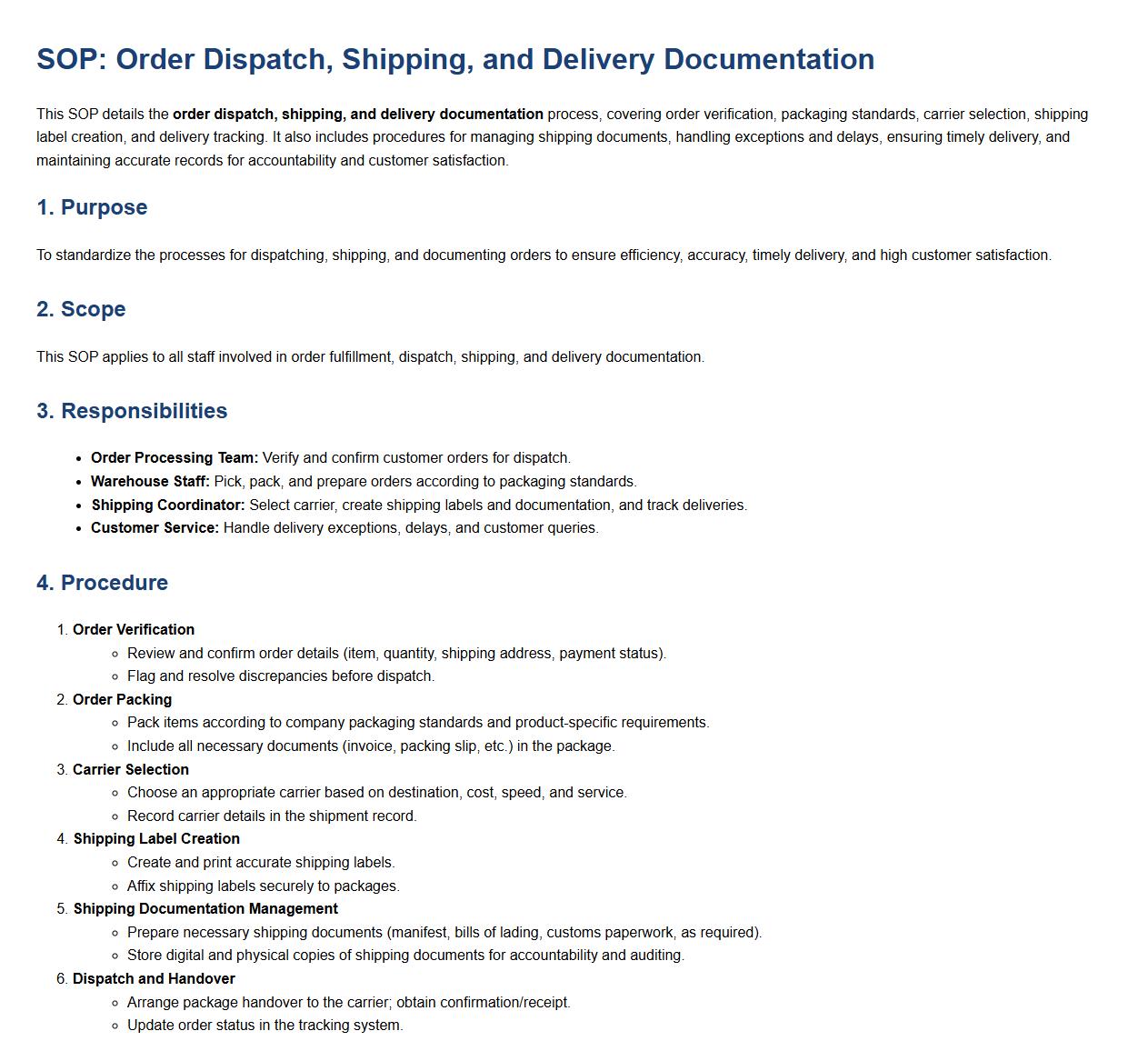
This SOP details the order dispatch, shipping, and delivery documentation process, covering order verification, packaging standards, carrier selection, shipping label creation, and delivery tracking. It also includes procedures for managing shipping documents, handling exceptions and delays, ensuring timely delivery, and maintaining accurate records for accountability and customer satisfaction.
Post-shipment feedback and customer support follow-up.

This SOP details the process for post-shipment feedback and customer support follow-up, encompassing systematic collection of customer feedback after product delivery, timely response to customer inquiries and concerns, proactive resolution of issues, documentation of customer interactions, and continuous improvement based on customer input. The objective is to enhance customer satisfaction, build long-term relationships, and ensure the effectiveness of shipment and support services through attentive and responsive communication.
What key steps must be followed in the SOP for Manufacturing Order Processing to ensure compliance and efficiency?
The SOP for Manufacturing Order Processing mandates precise order verification to ensure accuracy before production begins. It requires systematic material allocation and scheduling to optimize workflow and minimize delays. Additionally, strict adherence to quality control checkpoints ensures compliance with industry standards and operational efficiency.
Which departments or roles are responsible for each stage outlined in the Manufacturing Order Processing SOP?
The Production Planning team initiates the manufacturing order and schedules workflow stages. The Quality Assurance department oversees inspections and compliance checks throughout the process. Meanwhile, the Warehouse team manages material preparation and final product storage, ensuring seamless coordination across all stages.
What documentation and approvals are required at critical control points in the Manufacturing Order Processing SOP?
At critical control points, production orders must be documented and approved by the Production Manager to validate authorization. Quality control reports are mandatory to confirm compliance with standards before proceeding to the next stage. Additionally, material usage logs require sign-off by Inventory Control to maintain traceability and accuracy.
How does the SOP address handling of discrepancies or deviations during the order processing workflow?
The SOP specifies immediate reporting of discrepancies to the Quality Assurance department for swift investigation. Corrective actions must be documented and authorized before production continuation to mitigate risks. Furthermore, the deviation records are reviewed in regular management meetings to implement process improvements.
What records and logs must be maintained according to the SOP for Manufacturing Order Processing for audit and traceability?
The SOP requires maintaining comprehensive production logs detailing each step, timestamp, and responsible personnel. Quality inspection records with approval stamps are essential for audit trails. Finally, material usage and inventory adjustment logs must be preserved to ensure full traceability and regulatory compliance.
