
A SOP Template for Receiving Goods in Manufacturing ensures a standardized process for inspecting and verifying incoming materials to maintain quality control. It outlines key steps such as checking delivery documents, inspecting item conditions, and updating inventory records accurately. Following this template minimizes errors, reduces delays, and enhances overall supply chain efficiency.
Pre-receipt notification and scheduling.

This SOP details the pre-receipt notification and scheduling process, ensuring timely communication and coordination between suppliers and receiving departments. It covers procedures for advance notice of incoming shipments, scheduling delivery times to optimize workflow, confirming shipment details, and managing any necessary adjustments. The objective is to streamline receipt operations, minimize delays, and enhance overall supply chain efficiency.
Verification of delivery documents (purchase order, invoice, packing list).

This SOP details the verification of delivery documents including purchase orders, invoices, and packing lists. It ensures the accuracy and consistency of all delivered goods by matching delivery documents against original orders, confirming quantities, item descriptions, and prices. This process helps prevent discrepancies, supports proper inventory management, and facilitates timely payment processing while maintaining thorough documentation and accountability within the supply chain.
Physical inspection of goods upon arrival.

This SOP details the physical inspection of goods upon arrival, focusing on verifying the quantity, quality, and condition of received items against purchase orders and shipping documents. It ensures all goods meet specified standards, identifies damaged or non-compliant products immediately, and facilitates accurate record-keeping to maintain inventory integrity and support efficient supply chain management.
Unloading procedures and use of appropriate equipment.
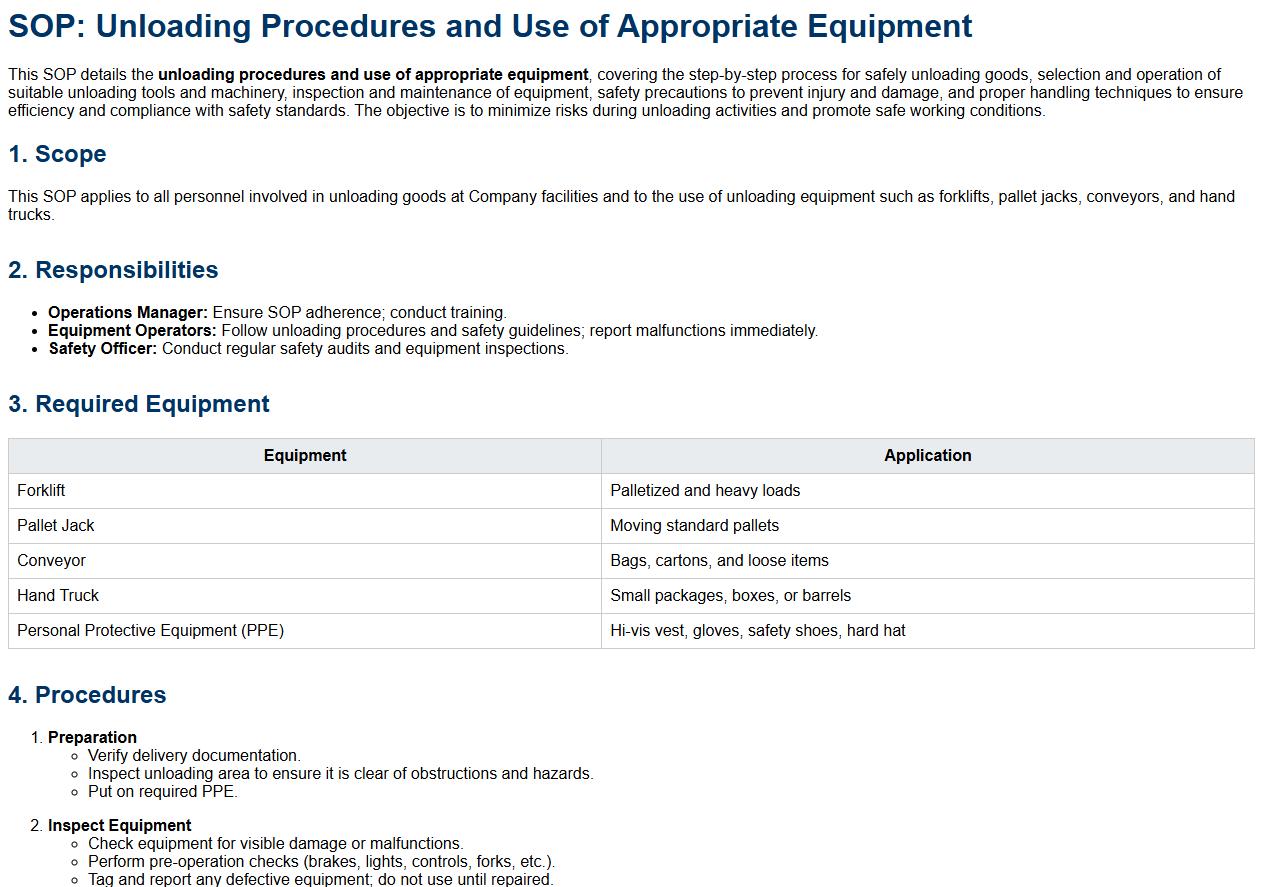
This SOP details the unloading procedures and use of appropriate equipment, covering the step-by-step process for safely unloading goods, selection and operation of suitable unloading tools and machinery, inspection and maintenance of equipment, safety precautions to prevent injury and damage, and proper handling techniques to ensure efficiency and compliance with safety standards. The objective is to minimize risks during unloading activities and promote safe working conditions.
Quantity and quality checks against purchase order/specifications.

This SOP details the process for quantity and quality checks against purchase order/specifications, ensuring that all received goods conform to the required standards and ordered quantities. It covers steps for inspecting products upon receipt, verifying compliance with purchase order specifications, documenting discrepancies, and coordinating with suppliers for resolution to maintain product integrity and operational efficiency.
Documentation and reporting of damages or discrepancies.

This SOP details the process for documentation and reporting of damages or discrepancies, including timely identification, accurate recording, verification procedures, communication protocols, and follow-up actions. Its aim is to ensure transparency, accountability, and prompt resolution of issues to maintain operational efficiency and minimize risks.
Goods labeling and tagging procedures.

This SOP defines goods labeling and tagging procedures to ensure accurate identification, tracking, and handling of products throughout the supply chain. It covers the standards for label content, format, placement, and durability, as well as protocols for barcode and RFID tag usage, inspection, and documentation. The goal is to facilitate effective inventory management, prevent errors, and comply with regulatory requirements.
Safe storage and segregation of received materials.

This SOP details the procedures for the safe storage and segregation of received materials, ensuring materials are properly identified, categorized, and stored to prevent contamination, damage, and safety hazards. It covers guidelines for handling different types of materials, storage conditions, segregation protocols to avoid cross-contamination, and regular inspection and maintenance of storage areas to maintain optimal safety and compliance with regulatory standards.
Updating inventory management systems.

This SOP details the process for updating inventory management systems, ensuring accurate tracking of stock levels, timely recording of new arrivals and withdrawals, synchronization between physical and digital inventories, regular data validation and error correction, implementation of system upgrades, and staff training for efficient system use. The goal is to maintain reliable inventory data to support effective supply chain management, reduce stock discrepancies, and enhance overall operational efficiency.
Communication and coordination with relevant departments (procurement, quality, warehouse, finance).

This SOP establishes clear guidelines for communication and coordination with relevant departments, including procurement, quality, warehouse, and finance. It details the processes for timely information exchange, cross-department collaboration, and unified decision-making to enhance operational efficiency, maintain quality standards, optimize inventory management, and ensure accurate financial tracking. The SOP aims to foster transparent communication channels and coordinated efforts among departments to achieve organizational goals effectively.
What are the key steps outlined in the SOP for verifying the quantity and quality of received goods?
The SOP emphasizes initial inspection of all goods upon arrival to verify their quantity matches the purchase order. It mandates a detailed quality check to ensure products meet specified standards and are free from defects. Finally, the procedure requires documentation of all findings to maintain accurate records.
Who is responsible, according to the SOP, for inspecting and documenting the condition of incoming shipments?
The SOP assigns the responsibility to the receiving department personnel for inspecting all incoming shipments. These designated individuals must carefully document any damages or discrepancies encountered during the process. Accountability is stressed to maintain integrity in the goods receipt process.
What actions does the SOP prescribe if discrepancies or damages are found during goods receipt?
When discrepancies or damage are identified, the SOP requires immediate notification to the supplier and the purchasing department. A formal report documenting the issue must be filed within a specific timeframe. Corrective actions, such as return or replacement, are then initiated to resolve the issue promptly.
How does the SOP ensure proper traceability and recording of received materials in the inventory system?
The SOP mandates entry of all received materials into the inventory management system with detailed batch and lot numbers. This facilitates accurate tracking throughout the supply chain and supports efficient inventory control. Additionally, the system updates help with future audits and product recalls if necessary.
What safety and compliance checks are mandated in the SOP before accepting goods into the facility?
The SOP requires comprehensive safety inspections including verification of compliance with regulatory standards before goods are accepted. It also mandates assessment of hazardous material documentation and labeling for proper handling. Ensuring these checks helps maintain workplace safety and regulatory adherence.
