
A SOP Template for Manufacturing Quality Assurance provides a structured framework to ensure consistent production processes and compliance with industry standards. It outlines specific procedures for monitoring, controlling, and verifying product quality throughout the manufacturing cycle. This template helps streamline quality management, reduce errors, and maintain regulatory adherence effectively.
Raw material inspection and acceptance procedures.

This SOP details the raw material inspection and acceptance procedures, including the criteria for quality assessment, documentation requirements, sampling methods, and rejection protocols. It aims to ensure that all raw materials meet specified standards before entering the production process, thereby maintaining product quality and compliance with regulatory requirements. The procedure covers supplier verification, visual and physical inspections, and the handling of non-conforming materials to minimize production disruptions and waste.
Standardized equipment calibration and maintenance routines.
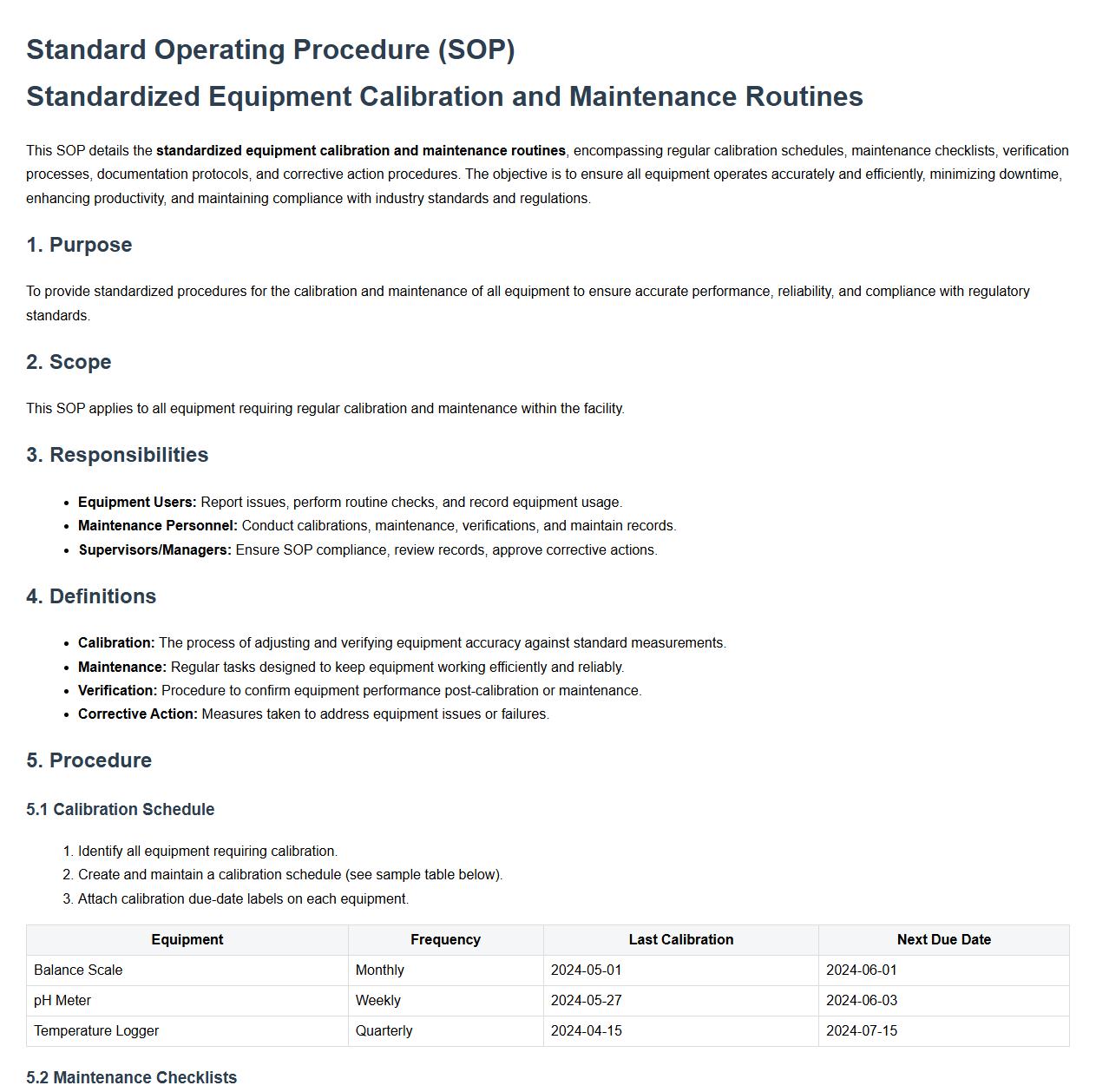
This SOP details the standardized equipment calibration and maintenance routines, encompassing regular calibration schedules, maintenance checklists, verification processes, documentation protocols, and corrective action procedures. The objective is to ensure all equipment operates accurately and efficiently, minimizing downtime, enhancing productivity, and maintaining compliance with industry standards and regulations.
In-process quality control checks and documentation.

This SOP details the procedures for in-process quality control checks and documentation, including systematic inspection points during production, criteria for acceptance and rejection, methods for recording observations, corrective action protocols, and maintaining accurate and comprehensive records. The objective is to ensure consistent product quality, prevent defects, and provide traceability throughout the manufacturing process.
Finished goods sampling and final inspection protocols.
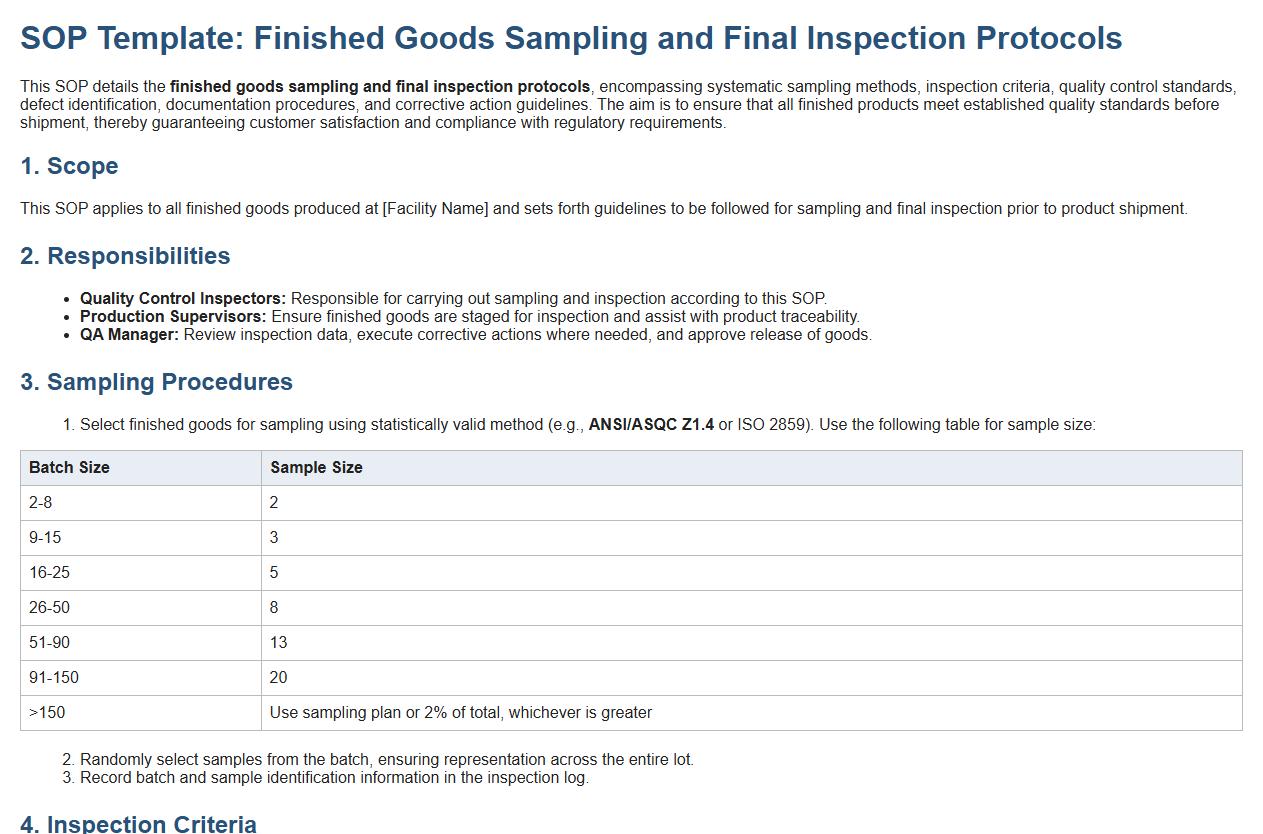
This SOP details the finished goods sampling and final inspection protocols, encompassing systematic sampling methods, inspection criteria, quality control standards, defect identification, documentation procedures, and corrective action guidelines. The aim is to ensure that all finished products meet established quality standards before shipment, thereby guaranteeing customer satisfaction and compliance with regulatory requirements.
Non-conformance identification and corrective action processes.

This SOP details the non-conformance identification and corrective action processes, including procedures for detecting deviations from standards, documenting non-conformities, analyzing root causes, implementing corrective and preventive actions, monitoring effectiveness, and maintaining records. The aim is to ensure continuous improvement and compliance with quality management systems by promptly addressing and rectifying any discrepancies.
Batch record completion and review guidelines.
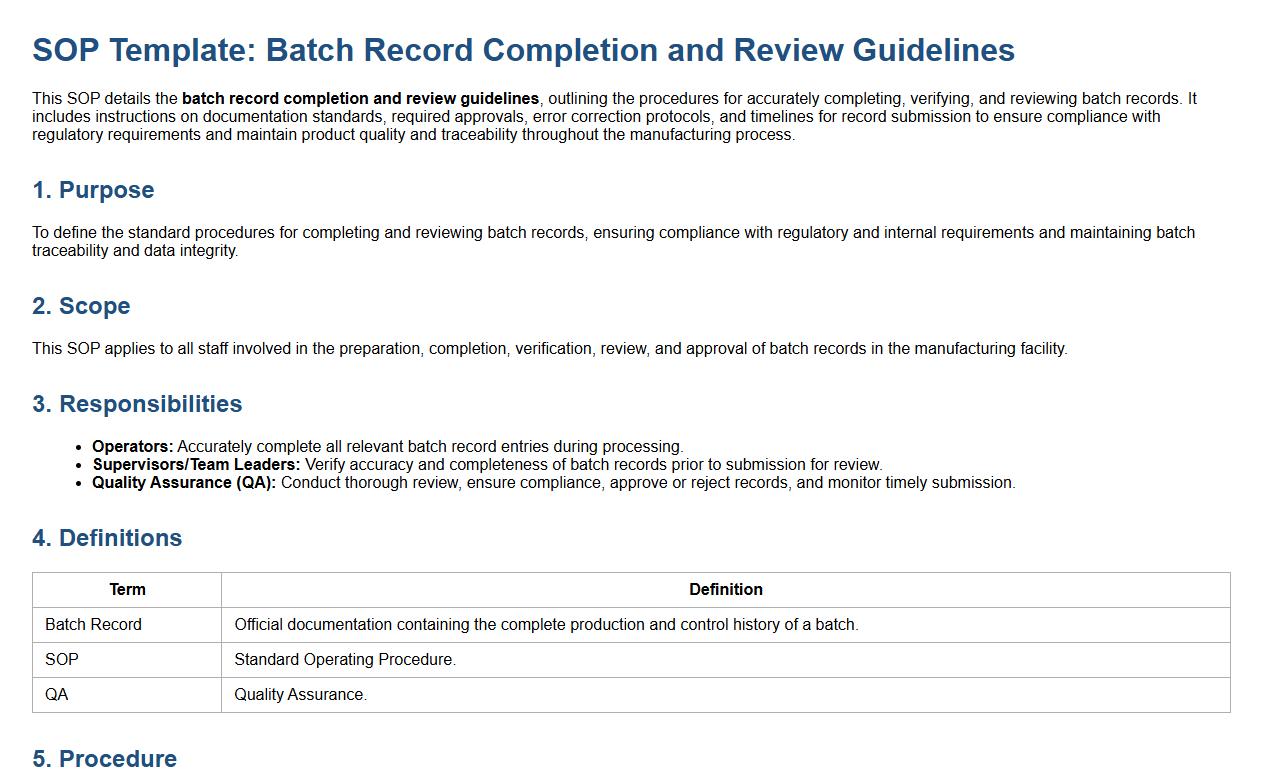
This SOP details the batch record completion and review guidelines, outlining the procedures for accurately completing, verifying, and reviewing batch records. It includes instructions on documentation standards, required approvals, error correction protocols, and timelines for record submission to ensure compliance with regulatory requirements and maintain product quality and traceability throughout the manufacturing process.
Change control request and approval procedures.
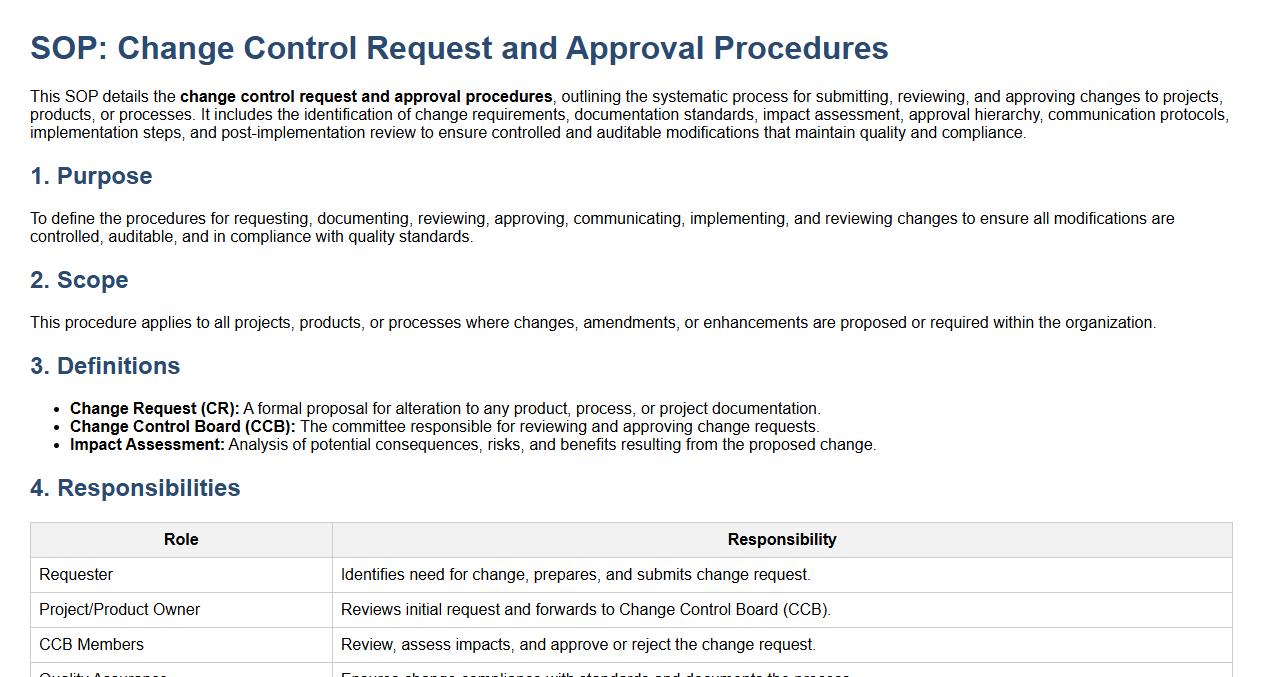
This SOP details the change control request and approval procedures, outlining the systematic process for submitting, reviewing, and approving changes to projects, products, or processes. It includes the identification of change requirements, documentation standards, impact assessment, approval hierarchy, communication protocols, implementation steps, and post-implementation review to ensure controlled and auditable modifications that maintain quality and compliance.
Product labeling and traceability management.

This SOP details product labeling and traceability management processes, encompassing accurate labeling requirements, batch and lot number assignment, barcode implementation, record-keeping protocols, compliance with regulatory standards, and procedures for tracking products through the supply chain. The objective is to ensure product integrity, facilitate efficient recalls, enhance quality control, and maintain transparent and reliable traceability from production to end consumer.
Complaint handling and root cause analysis workflows.
This SOP defines the complaint handling and root cause analysis workflows, detailing the systematic process for receiving, documenting, investigating, and resolving customer complaints. It ensures prompt identification of underlying issues through thorough root cause analysis, enabling effective corrective actions and continuous improvement. The SOP aims to enhance customer satisfaction, maintain regulatory compliance, and prevent recurrence of problems by fostering transparent communication and structured problem-solving methodologies.
Employee training and competency evaluation requirements.

This SOP details the employee training and competency evaluation requirements, covering the procedures for identifying training needs, delivering effective training programs, assessing employee skills and knowledge, maintaining training records, and ensuring continuous competency development. The goal is to enhance workforce capabilities, ensure compliance with regulatory standards, and promote a culture of safety and efficiency within the organization.
What are the key objectives outlined in the Manufacturing Quality Assurance SOP?
The primary objectives of the Manufacturing Quality Assurance SOP are to ensure consistent product quality and maintain compliance with industry standards. It aims to minimize defects and improve process efficiency through stringent quality controls. Additionally, the SOP focuses on safeguarding consumer safety by promoting a culture of continuous improvement.
Which critical control points are identified and managed according to the SOP?
The SOP identifies critical control points such as raw material inspection, in-process testing, and final product evaluation. Each control point is managed through predefined criteria and monitoring procedures to prevent quality deviations. This proactive approach helps in early detection and mitigation of potential risks in the manufacturing process.
What procedures does the SOP specify for documenting deviations and corrective actions?
The SOP mandates detailed documentation of any deviations from established quality standards, including the nature and cause of the issue. It outlines steps for initiating corrective actions, conducting root cause analysis, and implementing preventive measures. Proper records ensure traceability and support continuous quality improvement efforts.
How does the SOP ensure compliance with relevant regulatory and quality standards?
The SOP integrates requirements from regulatory bodies and industry quality standards into all manufacturing processes. Regular audits and reviews are conducted to verify adherence and identify areas for enhancement. Training programs and updated guidelines ensure that staff remain knowledgeable about compliance obligations.
What roles and responsibilities are defined for personnel in maintaining manufacturing quality?
The SOP clearly defines roles and responsibilities for all personnel involved in the manufacturing process, emphasizing accountability at every level. Quality assurance teams are tasked with monitoring compliance, while operators must follow standardized procedures diligently. Management is responsible for providing resources and fostering a quality-centric environment.
