
A SOP Template for Cleaning and Sanitation in Manufacturing ensures consistent hygiene practices by outlining step-by-step procedures for cleaning equipment and work areas. This template helps maintain compliance with regulatory standards and reduces the risk of contamination in production processes. Clear documentation supports employee training and promotes a safe, efficient manufacturing environment.
Cleaning and sanitation scheduling and frequency.
This SOP defines the cleaning and sanitation scheduling and frequency to maintain hygiene and prevent contamination. It includes establishing routine cleaning intervals, specifying cleaning methods and agents, assigning responsibilities, monitoring compliance, and documenting procedures to ensure a consistent and effective sanitation program in the facility.
Pre-cleaning inspection and preparation checklist.

This SOP details the pre-cleaning inspection and preparation checklist, covering the thorough assessment of equipment and surfaces before cleaning, identification of potential hazards, verification of cleaning tools and supplies, and proper safety precautions. The objective is to ensure an efficient, safe, and effective cleaning process by systematically preparing the environment and personnel prior to cleaning activities.
Proper equipment shutdown and lockout/tagout procedures.

This SOP details the proper equipment shutdown and lockout/tagout procedures to ensure the safe de-energization and isolation of machinery during maintenance or repair. It includes steps for notifying affected personnel, shutting down equipment, applying lockout/tagout devices, verifying energy isolation, and following safe startup protocols. The objective is to prevent accidental equipment startup, protect workers from hazardous energy, and maintain workplace safety throughout maintenance activities.
Selection and preparation of cleaning agents and sanitizers.

This SOP details the selection and preparation of cleaning agents and sanitizers, covering criteria for choosing effective and safe products, proper dilution methods, compatibility with surfaces and equipment, storage requirements, and safety precautions to ensure optimal hygiene standards and prevent contamination in various environments.
Step-by-step cleaning process for equipment and surfaces.
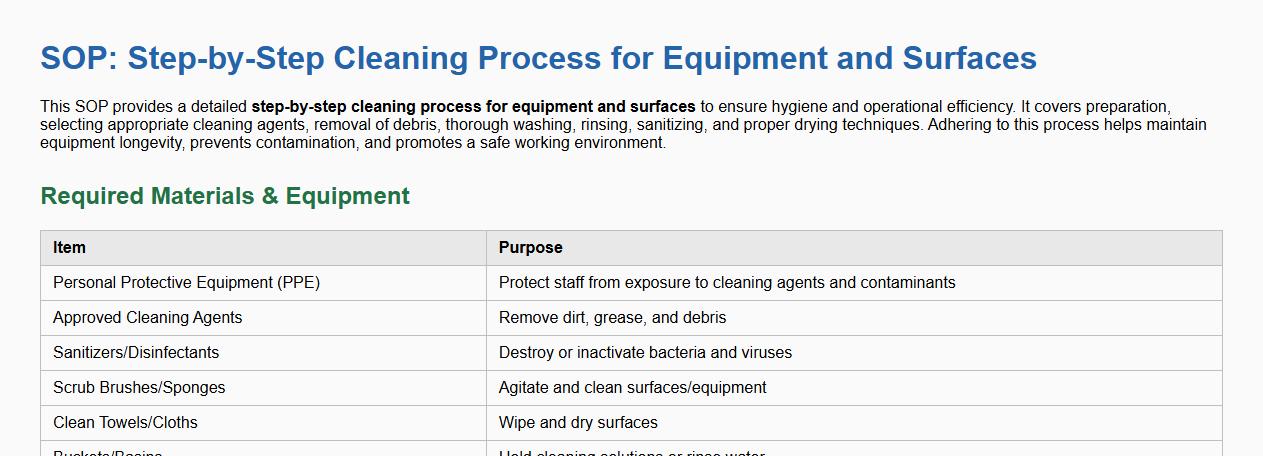
This SOP provides a detailed step-by-step cleaning process for equipment and surfaces to ensure hygiene and operational efficiency. It covers preparation, selecting appropriate cleaning agents, removal of debris, thorough washing, rinsing, sanitizing, and proper drying techniques. Adhering to this process helps maintain equipment longevity, prevents contamination, and promotes a safe working environment.
Sanitation of high-touch and critical control areas.

This SOP details the sanitation of high-touch and critical control areas, encompassing standardized cleaning protocols, frequency of sanitation procedures, approved disinfectants and cleaning agents, proper use of cleaning equipment, staff responsibilities, and monitoring and verification processes. The objective is to minimize contamination risks, prevent the spread of pathogens, and maintain a safe and hygienic environment in sensitive operational zones.
Post-cleaning inspection and verification procedures.

This SOP details the post-cleaning inspection and verification procedures, encompassing the systematic evaluation of cleanliness levels, identification of residual contaminants, documentation of inspection results, and verification protocols to ensure compliance with hygiene standards. It aims to maintain a safe and sanitary environment by confirming that cleaning processes have been effectively completed before equipment or areas are returned to operational use.
Waste disposal and management guidelines.

This SOP provides comprehensive waste disposal and management guidelines to ensure the safe, efficient, and environmentally responsible handling of waste materials. It covers waste segregation, collection, storage, transportation, treatment, and final disposal processes. The guidelines aim to minimize environmental impact, comply with regulatory requirements, promote recycling and resource recovery, and protect public health and safety through proper waste management practices.
Personal protective equipment (PPE) usage and hygiene requirements.
This SOP defines the standards for personal protective equipment (PPE) usage and hygiene requirements, ensuring proper selection, correct usage, maintenance, and disposal of PPE to protect employees from workplace hazards. It covers protocols for PPE inspection, cleaning, storage, and hygiene practices to prevent contamination and enhance worker safety and health across all work environments.
Documentation, record-keeping, and corrective action protocol.

This SOP establishes the documentation, record-keeping, and corrective action protocol, ensuring accurate and systematic recording of operational activities, incidents, and audit findings. It details procedures for maintaining organized records, timely documentation of non-conformities, and implementing corrective actions to address and prevent recurrence. The objective is to enhance transparency, facilitate compliance with regulatory requirements, and drive continuous improvement within the organization.
What are the primary objectives outlined in the SOP for cleaning and sanitation in manufacturing?
The primary objectives of the SOP are to ensure a safe and hygienic manufacturing environment by preventing contamination. It focuses on protecting product quality and maintaining compliance with regulatory standards. Additionally, the SOP aims to standardize cleaning processes to enhance operational efficiency.
Which specific areas and equipment does the SOP mandate for routine cleaning and sanitation?
The SOP mandates routine cleaning of critical production areas, including processing zones, storage rooms, and packaging lines. It also specifies cleaning of essential equipment such as mixers, conveyors, and filling machines. Emphasis is placed on high-contact surfaces to avoid cross-contamination risks.
What frequency and schedule does the SOP require for each cleaning and sanitation task?
The SOP defines specific cleaning frequencies based on the area and equipment usage, including daily, weekly, and monthly schedules. High-risk zones require immediate cleaning after each production cycle, while less critical areas follow periodic schedules. This structured timetable ensures consistent sanitation and prevents bacterial buildup.
Who is responsible, according to the SOP, for carrying out and recording cleaning and sanitation activities?
The SOP assigns responsibility to trained sanitation staff and production personnel for executing cleaning tasks. Supervisors oversee these activities and ensure proper documentation. Accountability is reinforced through detailed records of who performed cleaning and at what time.
What documentation and verification steps are described in the SOP to ensure compliance and effectiveness?
The SOP requires detailed cleaning logs and checklists to document all sanitation activities systematically. Verification includes routine inspections and microbial testing to confirm cleanliness levels. Corrective actions must be recorded in case of deviations to maintain continuous compliance.
