
A SOP Template for Process Documentation in Manufacturing ensures consistent and clear guidelines for operational procedures, improving efficiency and compliance. This template standardizes documentation, making it easier to train employees and maintain quality control. Implementing a well-structured SOP template minimizes errors and streamlines production workflows.
Process identification and scope definition.

This SOP provides a detailed framework for process identification and scope definition, outlining steps to accurately identify key processes within an organization and clearly define their boundaries and objectives. It includes guidelines for analyzing process inputs, outputs, stakeholders, and resources, ensuring alignment with organizational goals and facilitating effective process management and improvement initiatives.
Process flowchart and step-by-step workflow.
This SOP provides a detailed process flowchart and step-by-step workflow designed to standardize tasks and improve operational efficiency. The flowchart visually represents each stage of the process, highlighting key decision points and actions, while the step-by-step workflow offers clear instructions to ensure consistency, accuracy, and timely completion of tasks. This structured approach supports effective communication, reduces errors, and facilitates training and process optimization across the organization.
Roles, responsibilities, and authority matrix.

This SOP defines the roles, responsibilities, and authority matrix within the organization, detailing the specific duties and decision-making powers assigned to each position. It aims to ensure clarity, accountability, and efficient workflow by outlining who is responsible for tasks, who has the authority to approve actions, and how communication flows between departments. This framework supports effective management, risk mitigation, and organizational alignment with strategic objectives.
Materials, tools, and equipment specification.

This SOP defines the materials, tools, and equipment specification standards, detailing the criteria for selection, quality requirements, maintenance protocols, and proper usage guidelines. It aims to ensure that all materials and equipment used meet safety and performance standards, enhancing operational efficiency and minimizing risks associated with faulty or inappropriate tools in the workplace.
Standardized work instructions and methods.

This SOP defines standardized work instructions and methods to ensure consistent, efficient, and high-quality task execution across all operations. It includes clear guidelines for developing, implementing, and maintaining uniform procedures, detailed step-by-step instructions for each task, roles and responsibilities, quality control measures, and continuous improvement practices. The goal is to minimize variability, enhance productivity, and maintain safety and compliance standards throughout the organization.
Quality assurance and inspection checkpoints.
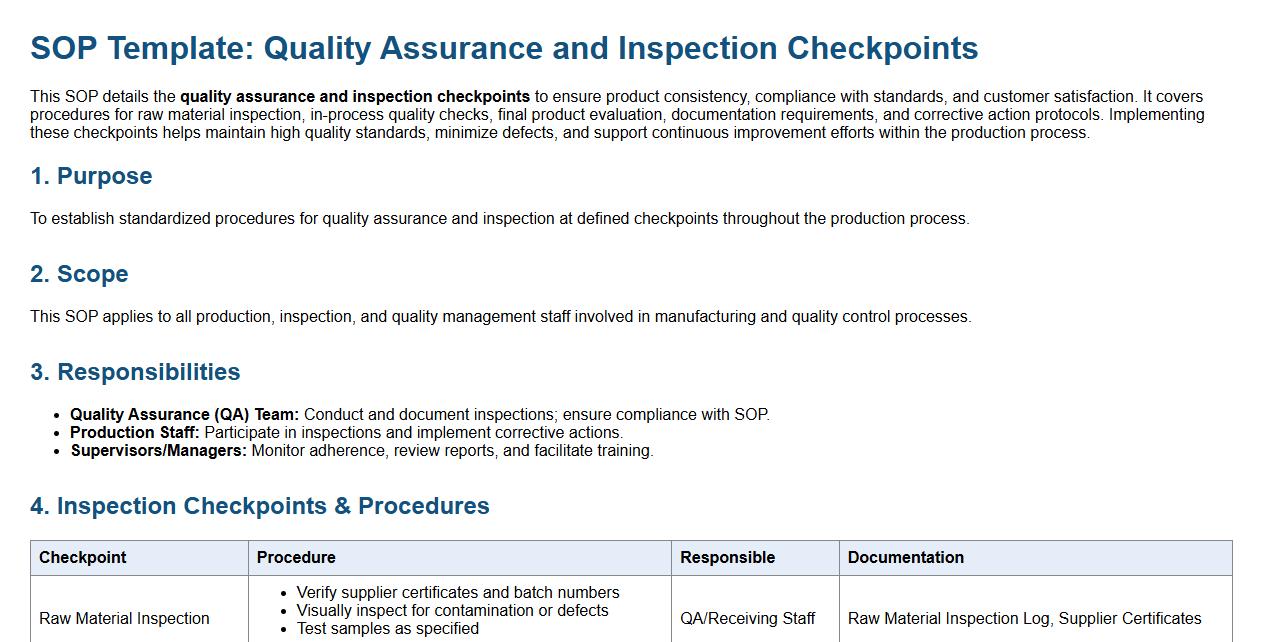
This SOP details the quality assurance and inspection checkpoints to ensure product consistency, compliance with standards, and customer satisfaction. It covers procedures for raw material inspection, in-process quality checks, final product evaluation, documentation requirements, and corrective action protocols. Implementing these checkpoints helps maintain high quality standards, minimize defects, and support continuous improvement efforts within the production process.
Health, safety, and environmental precautions.

This SOP details essential health, safety, and environmental precautions to protect employees, visitors, and the environment. It covers risk assessments, proper use of personal protective equipment (PPE), safe handling and disposal of hazardous materials, emergency response protocols, workplace ergonomics, and environmental conservation practices. The goal is to maintain a safe and healthy workplace while minimizing environmental impact through compliance with legal regulations and sustainable practices.
Non-conformance and corrective action procedures.

This SOP details the non-conformance and corrective action procedures, outlining the identification, documentation, investigation, and resolution of non-conformances. It establishes a systematic approach for addressing deviations from established standards, implementing corrective actions, verifying their effectiveness, and preventing recurrence. The goal is to ensure continuous improvement and maintain product quality and compliance with regulatory requirements.
Documentation, records, and version control.

This SOP describes the processes for documentation, records, and version control to ensure accurate, consistent, and secure management of all organizational documents. It covers guidelines for document creation, approval, distribution, storage, retrieval, and regular review. The procedure establishes version control protocols to track revisions, prevent unauthorized changes, and maintain historical records. Proper documentation and record keeping support compliance, accountability, and effective communication across departments.
Continuous improvement and review process.
This SOP details the continuous improvement and review process, encompassing regular evaluation of current practices, identification of areas for enhancement, implementation of improvement strategies, monitoring of outcomes, and systematic documentation. The aim is to foster a culture of ongoing development, ensuring that procedures remain effective, efficient, and aligned with organizational goals through structured feedback and iterative refinement.
What essential elements must be included in an effective SOP for process documentation in manufacturing?
An effective SOP for process documentation in manufacturing must include a clear purpose and scope to define its applicability. It should detail step-by-step procedures ensuring clarity and repeatability for manufacturing tasks. Additionally, safety guidelines and quality control measures are crucial to maintain compliance and product standards.
How does the SOP ensure consistency and compliance across all process documentation activities?
The SOP ensures consistency by standardizing documentation formats and terminology used within manufacturing processes. It mandates regular training for employees to maintain awareness and adherence to procedures. Compliance is reinforced through audits and reviews aligned with regulatory requirements and company policies.
What are the approval and revision procedures outlined in the SOP for updating process documents?
The approval procedure requires documentation to be reviewed and signed off by designated authorities before implementation. Revision procedures include scheduled periodic reviews and updates to reflect changes in processes or regulations. All changes must be documented with version control to track the history and rationale of updates.
How does the SOP address roles and responsibilities for creating, reviewing, and maintaining documents?
The SOP clearly defines roles for personnel responsible for drafting, reviewing, and approving process documents. It assigns accountability to specific departments or individuals for maintaining document accuracy and relevance. Continuous monitoring and feedback loops are established to ensure ongoing document integrity.
What procedures does the SOP specify for controlling access and distribution of process documentation?
The SOP specifies controlled access to sensitive process documentation through user permissions and secure storage systems. Distribution protocols ensure that only authorized personnel receive current and approved versions of documents. Additionally, obsolete copies are promptly retrieved and archived to prevent unauthorized use.
