
A SOP Template for Clinical Incident Reporting provides a standardized framework to document, analyze, and manage clinical incidents effectively within healthcare settings. This template ensures consistent recording of details such as the nature of the incident, involved parties, and corrective actions, promoting patient safety and regulatory compliance. Utilizing a clear SOP Template for Clinical Incident Reporting helps healthcare organizations streamline communication and improve quality of care.
Incident identification and initial response procedures.

This SOP details the incident identification and initial response procedures, covering the steps to recognize potential incidents promptly, assess the situation for safety hazards, initiate immediate response actions, notify appropriate personnel, and document the incident accurately. The objective is to ensure a swift and effective reaction to minimize harm, contain damage, and support subsequent investigation and resolution efforts.
Immediate patient safety measures and stabilization steps.

This SOP details the immediate patient safety measures and stabilization steps to be taken during emergency medical situations. It covers initial assessment, airway management, breathing and circulation support, prevention of further injury, vital sign monitoring, and preparation for advanced medical care. The goal is to ensure rapid intervention to protect patient well-being and stabilize their condition before transport or further treatment.
Incident notification and escalation guidelines.

This SOP defines incident notification and escalation guidelines to ensure timely and effective communication during emergencies or operational disruptions. It covers the identification of incidents, notification procedures, roles and responsibilities for reporting, escalation protocols based on incident severity, communication channels, and documentation requirements. The objective is to minimize impact by enabling prompt response, coordination, and resolution of incidents within the organization.
Accurate incident documentation requirements.

This SOP details the accurate incident documentation requirements, emphasizing the importance of timely and precise recording of all incidents within the workplace. It covers the necessary information to be documented, including date, time, location, individuals involved, description of the incident, and any immediate actions taken. The procedure ensures compliance with legal and organizational standards, facilitates effective incident analysis, supports claims and investigations, and promotes a safer work environment through proper record-keeping and communication.
Timelines and formats for report submission.
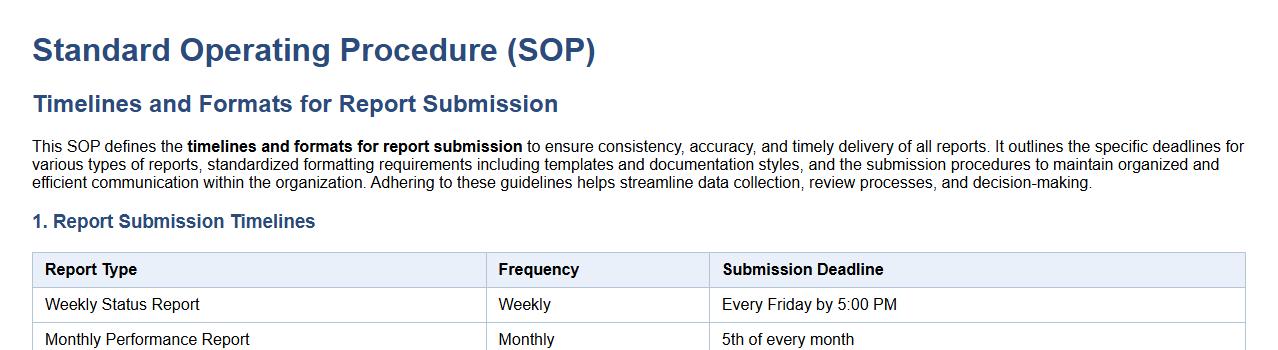
This SOP defines the timelines and formats for report submission to ensure consistency, accuracy, and timely delivery of all reports. It outlines the specific deadlines for various types of reports, standardized formatting requirements including templates and documentation styles, and the submission procedures to maintain organized and efficient communication within the organization. Adhering to these guidelines helps streamline data collection, review processes, and decision-making.
Roles and responsibilities of involved staff members.

This SOP defines the roles and responsibilities of involved staff members to ensure clarity and accountability within the organization. It outlines specific duties, reporting lines, and expectations for each team member to promote efficient collaboration and effective task execution. By clearly assigning responsibilities, the SOP aims to enhance operational workflows, improve communication, and support overall organizational goals.
Investigation and root cause analysis workflows.

This SOP details the investigation and root cause analysis workflows designed to systematically identify, analyze, and address the underlying causes of incidents and non-conformities. It encompasses steps for data collection, evidence evaluation, problem identification, and corrective action planning to prevent recurrence and promote continuous improvement within the organization.
Communication protocol for informing patients and families.

This SOP establishes a clear communication protocol for informing patients and families, detailing the standardized procedures for delivering timely, accurate, and compassionate information regarding patient status, treatment plans, and any changes in care. It emphasizes the importance of privacy, cultural sensitivity, and effective interpersonal communication to ensure that patients and their families are well-informed and supported throughout the healthcare process.
Corrective action and follow-up monitoring procedures.

This SOP details the corrective action and follow-up monitoring procedures necessary to identify, address, and resolve non-conformities or issues within operational processes. It covers the systematic steps for root cause analysis, implementing corrective measures, assigning responsibilities, and establishing timelines. The procedure also includes continuous monitoring and review to ensure effectiveness, prevent recurrence, and promote ongoing improvement in compliance and quality standards.
Confidentiality and data protection measures regarding incident reports.

This SOP details confidentiality and data protection measures concerning incident reports, emphasizing the secure handling, storage, and sharing of sensitive information. It includes guidelines on access control, data encryption, regular audits, compliance with legal and regulatory requirements, and staff training to ensure the privacy and integrity of incident data, thereby safeguarding individuals' rights and organizational trust.
What are the essential steps outlined in the SOP for reporting a clinical incident?
The SOP mandates immediate identification and assessment of the clinical incident to mitigate risks. It requires the prompt documentation of the incident details using standardized forms. Lastly, the SOP emphasizes timely communication to the appropriate departments for further investigation and resolution.
Who is responsible for initiating a clinical incident report according to the SOP?
The SOP assigns responsibility primarily to the healthcare professional who first identifies the incident. It also permits other staff members involved in patient care to initiate reports if they observe an incident. This ensures incidents are reported without delay, maintaining patient safety standards.
What information must be included in a clinical incident report as per the SOP?
According to the SOP, the report must document the date, time, and location of the incident. It should include detailed descriptions of what occurred, involved individuals, and any immediate actions taken. The SOP also requires noting the potential impact on patient safety and any witness statements.
What is the designated timeframe for submitting a clinical incident report under the SOP guidelines?
The SOP clearly states that clinical incident reports must be submitted within 24 hours of the occurrence. This prompt submission facilitates timely review and management of the incident. Delays in reporting are discouraged to uphold safety and quality care standards.
How does the SOP ensure confidentiality and data protection during clinical incident reporting?
The SOP enforces strict adherence to confidentiality protocols throughout the reporting process. It requires secure handling and storage of incident reports to protect patient and staff identities. Additionally, access to sensitive data is limited to authorized personnel only, ensuring compliance with privacy laws.
