
A SOP Template for Laboratory Test Procedures ensures consistent and accurate execution of testing protocols by providing clear, step-by-step instructions. This template helps standardize laboratory workflows, improve data reliability, and maintain compliance with regulatory standards. It is essential for training staff, maintaining quality control, and documenting all testing activities systematically.
Sample reception and labeling protocol.
This SOP details the sample reception and labeling protocol, covering procedures for receiving samples, verifying sample integrity, assigning unique identification codes, proper labeling techniques, and documentation requirements. The goal is to ensure accurate sample tracking, prevent contamination or mix-ups, and maintain the integrity of samples throughout handling and analysis processes.
Sample storage and preservation requirements.

This SOP details the sample storage and preservation requirements, covering proper labeling, temperature control, contamination prevention, and duration limits for different sample types. The aim is to maintain sample integrity, prevent degradation, and ensure reliable test results by following standardized procedures for handling, storing, and preserving biological, chemical, or environmental specimens.
Reagent preparation and quality control.

This SOP details the standardized procedures for reagent preparation and quality control, including the accurate measurement and mixing of chemical reagents, ensuring reagent purity and stability, calibration of equipment used, documentation of batch records, routine quality assessments, and corrective actions for non-conforming reagents. The goal is to maintain reagent consistency and reliability to support accurate experimental results and laboratory safety.
Equipment calibration and maintenance procedures.

This SOP describes equipment calibration and maintenance procedures, covering the systematic approach to regularly calibrate, inspect, and maintain equipment to ensure optimal performance, accuracy, and safety. It includes scheduling calibration intervals, documenting calibration records, performing preventive maintenance, troubleshooting common equipment issues, and establishing responsibilities for personnel involved. The goal is to minimize equipment downtime, extend service life, and maintain compliance with quality and safety standards.
Test method step-by-step workflow.

This SOP details the test method step-by-step workflow, including preparation of test materials, setup of equipment, execution of test procedures, data collection and recording, analysis of results, quality control checks, troubleshooting common issues, and documentation of findings. The goal is to ensure consistent, accurate, and reproducible test outcomes by following standardized processes and protocols throughout each testing phase.
Quality assurance and result validation guidelines.

This SOP provides comprehensive quality assurance and result validation guidelines designed to ensure the accuracy, reliability, and consistency of test results. It includes protocols for sample handling, calibration and maintenance of equipment, validation of analytical methods, documentation and review processes, corrective actions for discrepancies, and continuous improvement practices. The objective is to maintain high standards in laboratory operations, comply with regulatory requirements, and deliver trustworthy data for decision-making.
Waste disposal and biosafety measures.
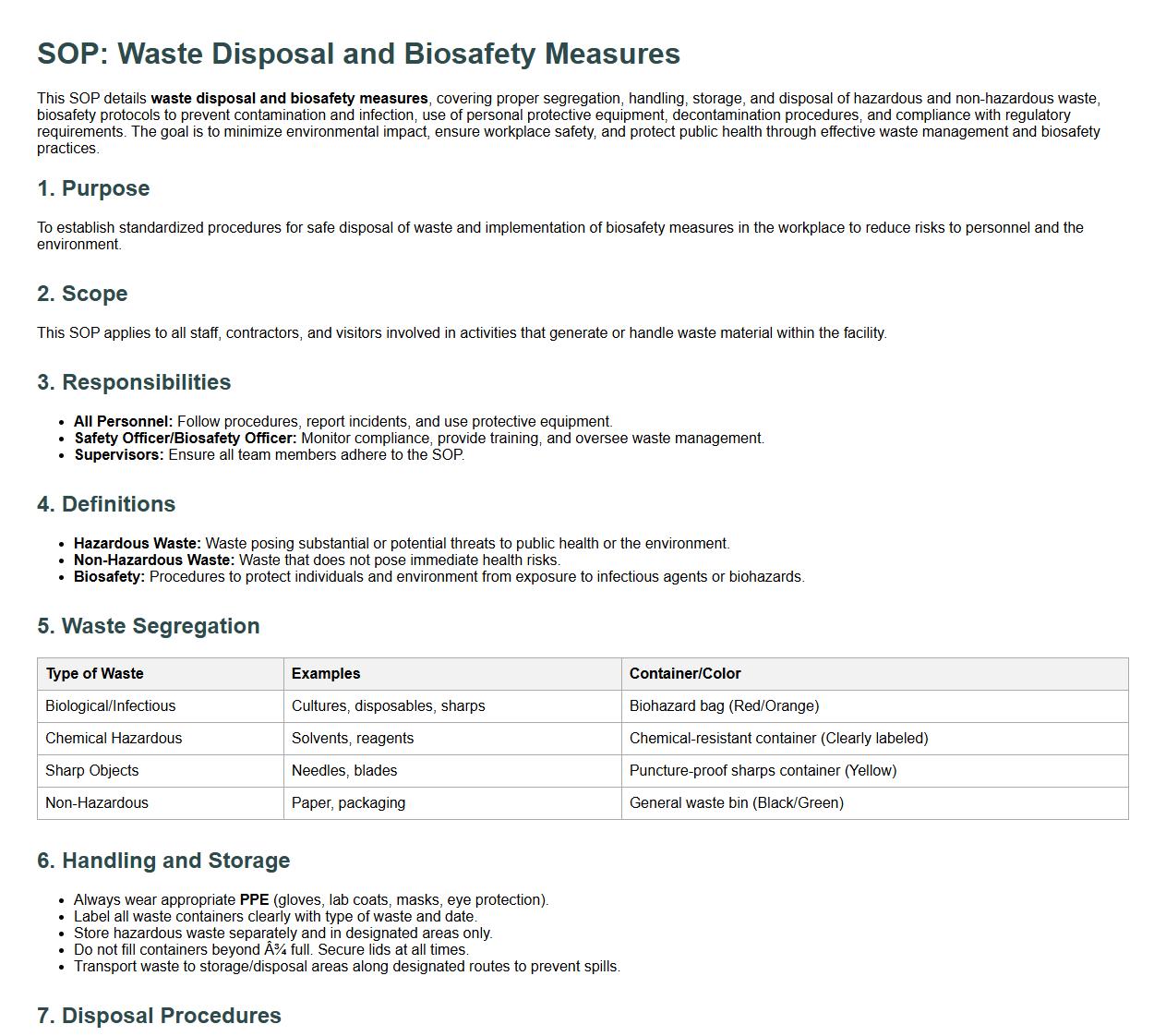
This SOP details waste disposal and biosafety measures, covering proper segregation, handling, storage, and disposal of hazardous and non-hazardous waste, biosafety protocols to prevent contamination and infection, use of personal protective equipment, decontamination procedures, and compliance with regulatory requirements. The goal is to minimize environmental impact, ensure workplace safety, and protect public health through effective waste management and biosafety practices.
Data recording and result documentation process.
This SOP details the data recording and result documentation process, encompassing systematic collection, accurate entry, validation, and secure storage of data, as well as comprehensive documentation of results. It aims to ensure data integrity, traceability, and compliance with regulatory standards, facilitating reliable analysis and informed decision-making throughout the project lifecycle.
Reporting and communication of test results.

This SOP details the reporting and communication of test results, covering standardized procedures for accurately documenting, reviewing, and disseminating test outcomes to relevant stakeholders. It ensures timely, clear, and confidential communication of results to support informed decision-making, maintain data integrity, uphold compliance with regulatory requirements, and facilitate appropriate follow-up actions.
Corrective actions for non-conformance or test failure.

This SOP details the process for identifying and implementing corrective actions for non-conformance or test failure. It covers the steps for detecting deviations, documenting discrepancies, analyzing root causes, developing and applying corrective measures, and verifying effectiveness to prevent recurrence. The objective is to ensure product quality, compliance with standards, and continuous improvement within the organization.
What is the primary objective of the SOP for Laboratory Test Procedures?
The primary objective of the SOP is to ensure consistent and accurate execution of laboratory tests. It aims to standardize procedures to maintain data integrity and reproducibility. This helps in achieving reliable results that support research and quality assurance.
Which specific laboratory tests are covered within this SOP?
The SOP covers a wide range of standard laboratory tests including chemical, biological, and physical analyses. Tests such as pH measurement, microbial assays, and titration procedures are explicitly included. This comprehensive coverage ensures all relevant tests follow uniform protocols.
What are the required safety measures outlined in the SOP during test procedures?
Strict safety measures are mandated, including the use of personal protective equipment (PPE) like gloves, goggles, and lab coats. The SOP also specifies handling hazardous materials carefully and adhering to proper waste disposal methods. These precautions minimize risks of accidents and contamination.
How does the SOP specify the documentation and reporting of laboratory results?
The SOP requires thorough documentation of all test procedures, observations, and outcomes in laboratory notebooks or digital logs. Results must be reported in a clear, concise format and reviewed for accuracy before submission. This ensures traceability and accountability in laboratory operations.
What are the criteria for equipment calibration and maintenance according to the SOP?
Regular calibration and maintenance of laboratory equipment are emphasized to guarantee precision and reliability. The SOP outlines schedules for routine checks, calibration against standards, and prompt repair of faulty instruments. Proper upkeep prevents errors and extends the lifespan of equipment.
