
A SOP Template for Operating Room Preparation ensures a standardized process for sterilization, equipment setup, and environment safety checks before surgery. It outlines step-by-step guidelines to maintain infection control and operational efficiency. This template helps healthcare teams minimize errors and enhance patient safety in the operating room.
Pre-operative room cleaning and disinfection procedures.

This SOP details the pre-operative room cleaning and disinfection procedures, emphasizing the importance of maintaining a sterile environment to prevent surgical site infections. It covers step-by-step cleaning protocols, selection and use of appropriate disinfectants, handling and disposal of waste, verification of surface cleanliness, and adherence to infection control standards. The goal is to ensure the operating room is properly sanitized and safe for surgical procedures, protecting patient health and promoting optimal outcomes.
Verification of surgical instrument sterilization and availability.

This SOP details the process for verification of surgical instrument sterilization and availability, encompassing the inspection of sterilization indicators, documentation of sterilization cycles, ensuring the integrity of packaging, and confirming the timely availability of sterilized instruments for surgical procedures. The goal is to maintain patient safety by preventing infections through strict adherence to sterilization protocols and ensuring that all required instruments are ready and properly sterilized before use.
Setup and arrangement of surgical instruments and supplies.

This SOP details the proper setup and arrangement of surgical instruments and supplies, ensuring all necessary tools are sterilized, organized, and readily accessible for surgical procedures. It covers the preparation of the sterile field, correct placement of instruments based on surgery type, verification of supply inventories, and maintaining aseptic techniques to promote patient safety and surgical efficiency.
Functionality check of anesthesia and monitoring equipment.
This SOP details the functionality check of anesthesia and monitoring equipment, ensuring all devices are properly calibrated, operational, and safe prior to use. It covers pre-use inspection procedures, verification of system alarms, battery and power supply status, sensor accuracy, and troubleshooting protocols. The objective is to guarantee reliable performance of anesthesia and monitoring equipment to maintain patient safety and support effective clinical outcomes during medical procedures.
Surgical lighting positioning and operation verification.

This SOP details the surgical lighting positioning and operation verification process, ensuring optimal illumination during surgical procedures. It includes guidelines for adjusting lighting angles, verifying intensity and focus, checking equipment functionality, and maintaining sterility standards. The objective is to provide a clear, well-lit surgical field, enhancing visibility and patient safety while minimizing interruptions during operations.
Patient identification and surgical site confirmation protocol.

This SOP details the patient identification and surgical site confirmation protocol, encompassing steps for verifying patient identity, confirming the correct surgical site and procedure, utilizing standardized checklists and time-out procedures, and communicating effectively among surgical team members. The objective is to prevent wrong-patient, wrong-site, and wrong-procedure surgeries, ensuring patient safety and compliance with healthcare regulations.
Personal protective equipment (PPE) donning guidelines for staff.

This SOP provides detailed personal protective equipment (PPE) donning guidelines for staff, including the proper selection, inspection, and correct sequence for wearing PPE to ensure maximum safety and hygiene. It emphasizes the importance of staff adherence to PPE protocols to prevent workplace contamination and exposure to hazards, thereby maintaining a safe and compliant working environment.
Proper handling and disposal of biohazard materials.

This SOP details the proper handling and disposal of biohazard materials, including identification and classification of biohazardous waste, use of personal protective equipment (PPE), safe collection and segregation procedures, storage requirements, approved disposal methods, and compliance with regulatory guidelines. The goal is to protect personnel, the environment, and the community from exposure to harmful biological agents and ensure safe and effective biohazard waste management.
Final pre-surgery room inspection checklist.
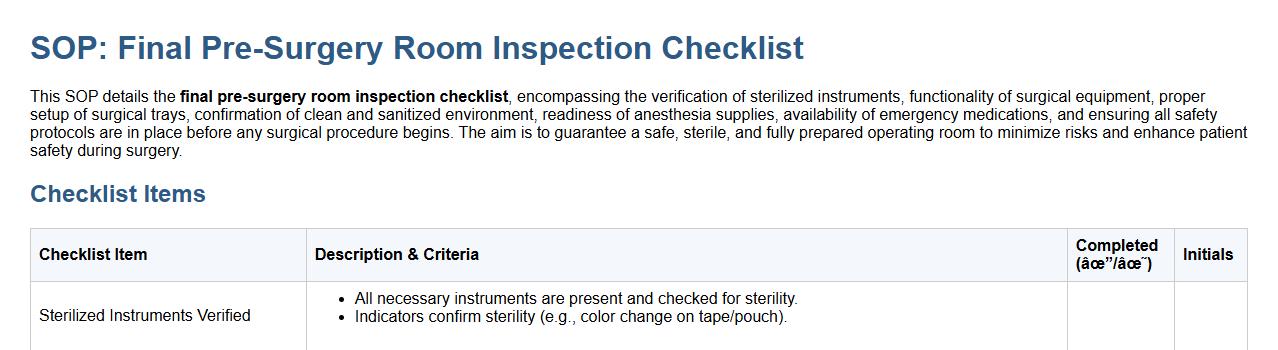
This SOP details the final pre-surgery room inspection checklist, encompassing the verification of sterilized instruments, functionality of surgical equipment, proper setup of surgical trays, confirmation of clean and sanitized environment, readiness of anesthesia supplies, availability of emergency medications, and ensuring all safety protocols are in place before any surgical procedure begins. The aim is to guarantee a safe, sterile, and fully prepared operating room to minimize risks and enhance patient safety during surgery.
Documentation and reporting of room preparation completion.

This SOP details the procedures for the documentation and reporting of room preparation completion, ensuring that all rooms are thoroughly prepared, inspected, and verified before use. It includes steps for recording preparation activities, verifying cleanliness and readiness, reporting completion status to relevant departments, and maintaining accurate records for quality control and accountability purposes.
What critical steps must be completed before beginning room sterilization in the SOP for Operating Room Preparation?
The SOP mandates a thorough cleaning of all surfaces and removal of debris before sterilization begins. It requires verification that all prior procedures and materials have been fully cleared from the room. Additionally, the environment must be checked for proper functioning of sterilization equipment to ensure effective room sterilization.
Which personnel roles are responsible for verifying the availability of surgical instruments according to the SOP?
The SOP assigns the responsibility of verifying surgical instrument availability primarily to the circulating nurse and surgical technician. These personnel must conduct a detailed instrument count and ensure all tools are sterile and accounted for. This verification process is critical to maintaining surgical efficiency and patient safety.
How does the SOP specify handling and storage of sterile supplies before a procedure?
The SOP requires sterile supplies to be handled with aseptic techniques to maintain sterility until use. Supplies must be stored in a designated sterile storage area, away from potential contaminants. Proper labeling and expiration date checks are essential to ensure readiness for the procedure.
What environmental controls are mandated by the SOP to ensure infection prevention in the Operating Room?
The SOP outlines stringent environmental controls including maintaining positive air pressure and frequent air filtration to reduce airborne contaminants. Temperature and humidity levels must be monitored and kept within specified limits. Strict adherence to these controls supports a sterile and safe surgical environment.
How does the SOP outline documentation requirements for preoperative room readiness?
The SOP specifies detailed documentation of all preparation activities before surgery, including equipment checks and room cleaning logs. It emphasizes the need for signatures from responsible personnel to verify completion. Accurate documentation ensures accountability and traceability in the preoperative process.
