
A SOP Template for Manufacturing Product Traceability ensures consistent tracking of products throughout the production process, enhancing quality control and compliance. This template outlines clear procedures for recording batch numbers, production dates, and movement logs, facilitating efficient recall management. Implementing this SOP strengthens accountability and streamlines traceability in manufacturing operations.
Product identification and lot/batch numbering system.
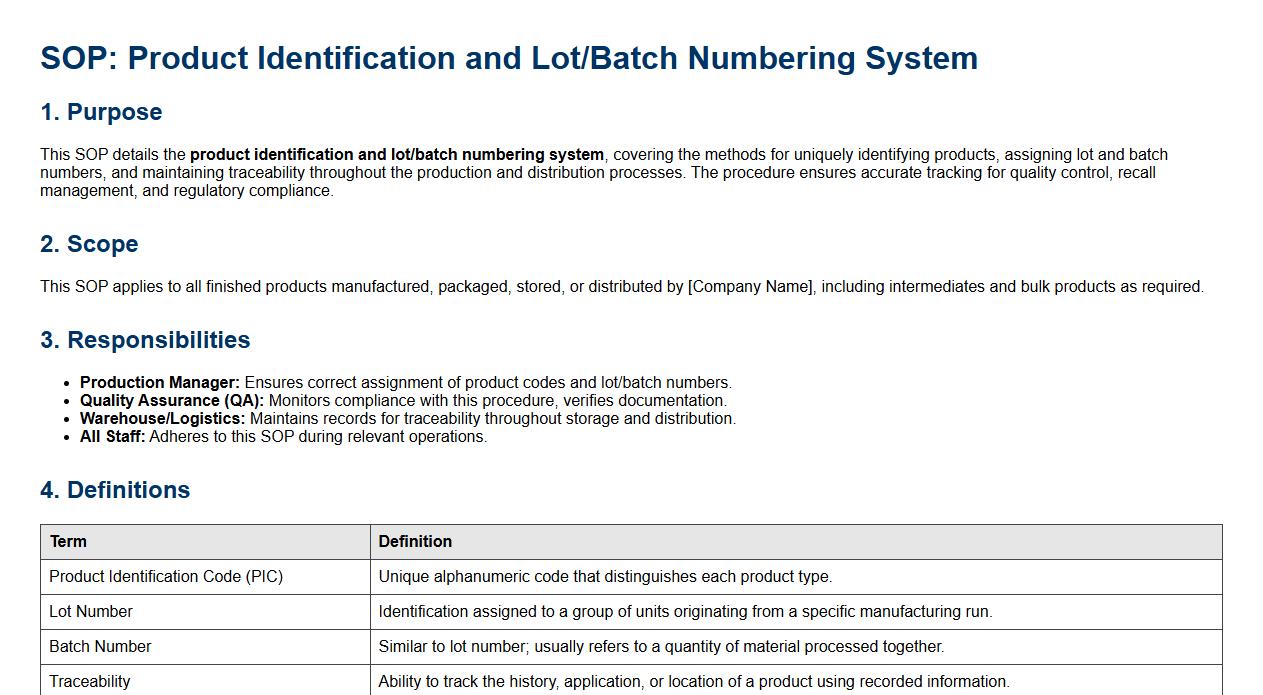
This SOP details the product identification and lot/batch numbering system, covering the methods for uniquely identifying products, assigning lot and batch numbers, and maintaining traceability throughout the production and distribution processes. The procedure ensures accurate tracking for quality control, recall management, and regulatory compliance.
Raw material receiving and inspection protocols.

This SOP defines the raw material receiving and inspection protocols, covering procedures for verifying supplier documentation, inspecting the quality and quantity of raw materials, handling and storage requirements, contamination prevention, non-conformance management, and record keeping. The goal is to ensure that all incoming raw materials meet predefined quality standards to maintain product integrity and regulatory compliance.
Component and material tracking through production stages.
This SOP details the process for component and material tracking through production stages, ensuring accurate monitoring and documentation from initial receipt to final assembly. It encompasses inventory management, barcode and RFID scanning protocols, stage-by-stage status updates, quality control checkpoints, and traceability measures. The objective is to maintain efficient workflow, minimize errors, enhance accountability, and support compliance with regulatory and quality standards throughout the manufacturing process.
Documentation and labeling procedures at each process step.

This SOP defines the documentation and labeling procedures at each process step to ensure accuracy, traceability, and compliance throughout the production or operational workflow. It includes guidelines for recording essential data, assigning clear and consistent labels, maintaining updated records, and verifying documentation integrity. The goal is to support efficient process tracking, quality control, and regulatory adherence by implementing standardized documentation and labeling practices at every stage.
In-process quality checks and recording requirements.

This SOP defines the in-process quality checks and recording requirements to ensure continuous monitoring and verification of product quality during manufacturing. It covers the procedures for conducting systematic inspections at various stages of production, criteria for quality acceptance, methods for documenting findings, and protocols for handling deviations. The objective is to maintain product consistency, identify defects early, and support traceability through accurate and timely record-keeping.
Finished goods packaging and labeling requirements.

This SOP defines the finished goods packaging and labeling requirements, covering packaging materials selection, proper labeling information, product identification, handling and storage instructions, compliance with regulatory standards, and quality assurance checks. The aim is to ensure that all finished products are securely packaged and accurately labeled to maintain product integrity, facilitate proper handling, and provide essential information to customers and distributors.
Shipping and distribution traceability documentation.

This SOP details the procedures for shipping and distribution traceability documentation, encompassing the accurate recording of shipment details, tracking of product movement through distribution channels, maintenance of traceability records, compliance with regulatory requirements, and ensuring swift identification of products in the event of recalls or quality issues. The objective is to guarantee transparency, accountability, and efficient management throughout the shipping and distribution process to uphold product integrity and customer satisfaction.
Record retention timeframe and storage protocols.
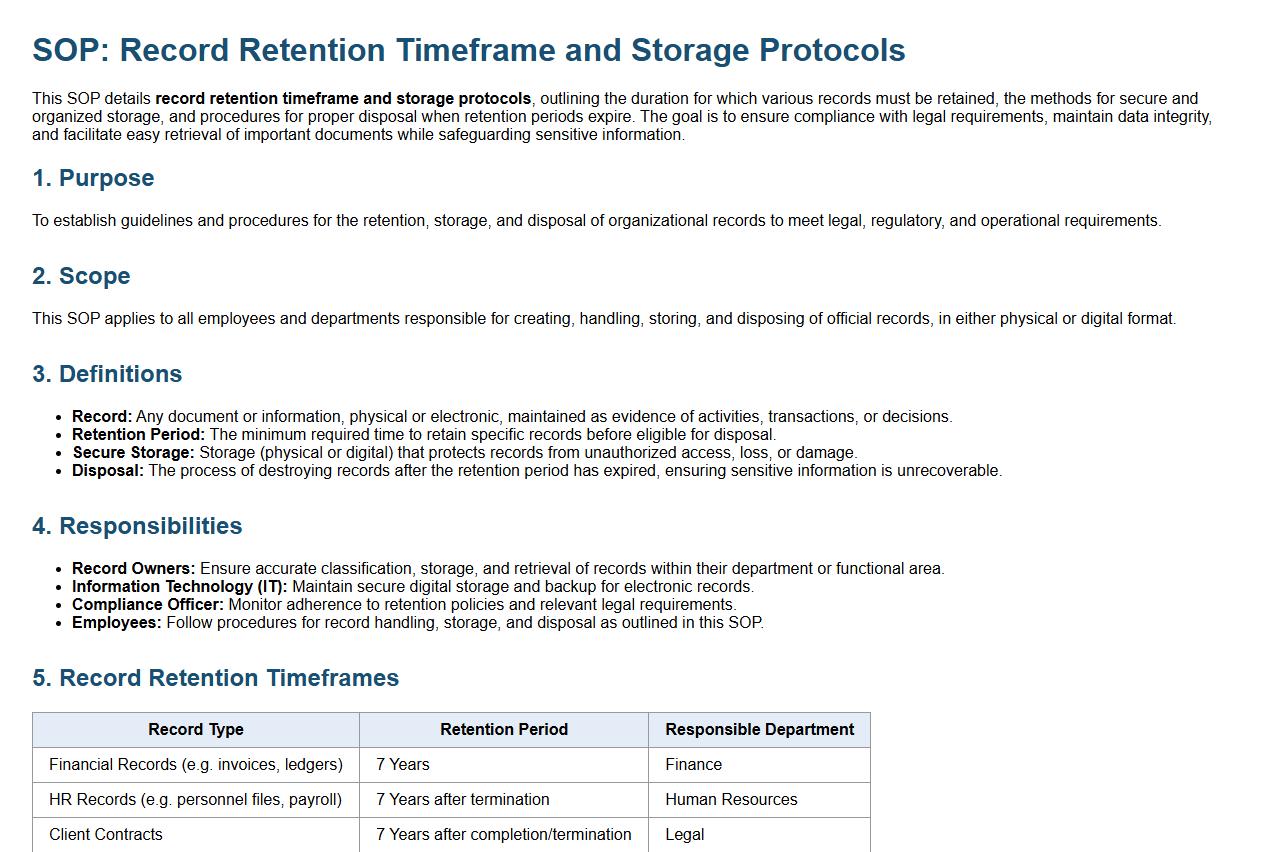
This SOP details record retention timeframe and storage protocols, outlining the duration for which various records must be retained, the methods for secure and organized storage, and procedures for proper disposal when retention periods expire. The goal is to ensure compliance with legal requirements, maintain data integrity, and facilitate easy retrieval of important documents while safeguarding sensitive information.
Recall and product withdrawal procedures.

This SOP details recall and product withdrawal procedures, covering the identification of affected products, communication protocols, coordination with regulatory authorities, customer notification, product retrieval processes, verification of corrective actions, and documentation requirements. The purpose is to ensure swift and effective removal of compromised products from the market to protect consumer safety and maintain regulatory compliance.
Internal audits and continuous improvement measures.
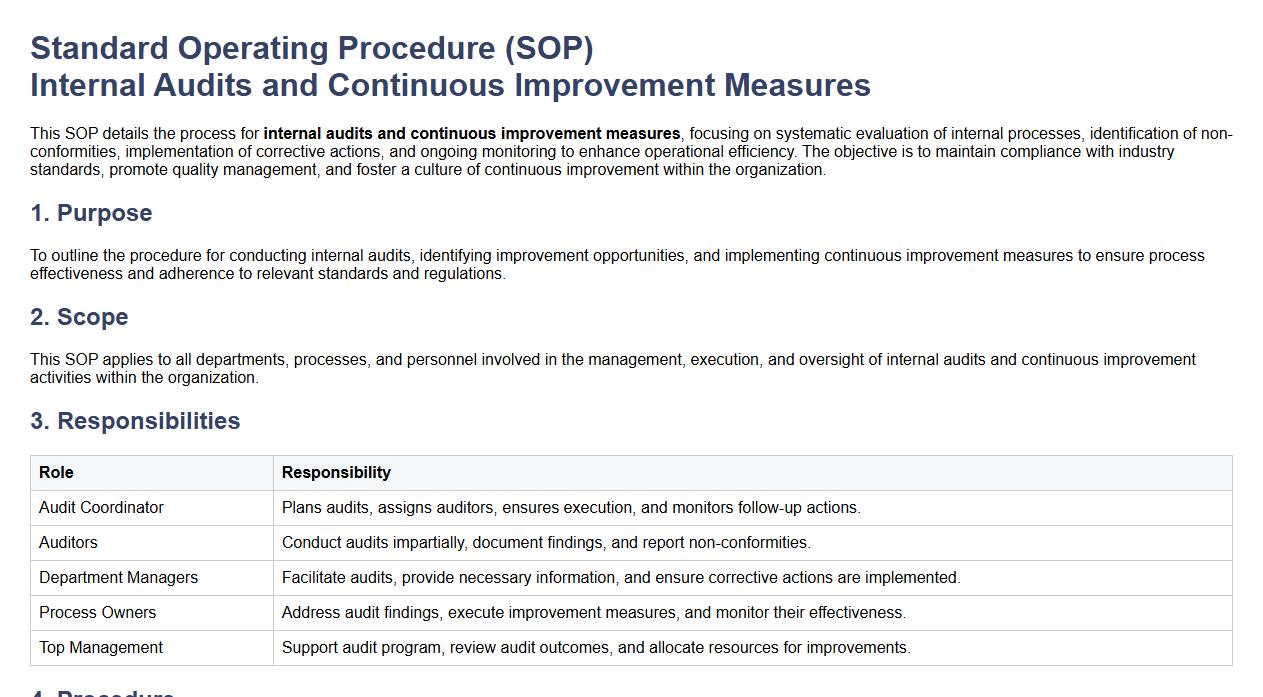
This SOP details the process for internal audits and continuous improvement measures, focusing on systematic evaluation of internal processes, identification of non-conformities, implementation of corrective actions, and ongoing monitoring to enhance operational efficiency. The objective is to maintain compliance with industry standards, promote quality management, and foster a culture of continuous improvement within the organization.
Key Data Elements to Record in Product Manufacturing
According to the traceability SOP, each stage of the product's manufacturing process must capture critical data elements such as raw material batch numbers, processing parameters, and personnel involved. This ensures a comprehensive record for quality control and accountability. Accurate recording of these elements supports effective root cause analysis if issues arise.
Definition and Assurance of Lot or Batch Identification
The SOP defines lot or batch identification as a unique code assigned to a group of products processed under similar conditions. This identification enables precise tracking from raw materials to finished goods. Strong procedures are established to guarantee the integrity and consistency of batch IDs throughout production.
Procedures for Handling Discrepancies in Traceability Data
If a discrepancy is detected, the SOP mandates an immediate review and investigation by quality assurance. All findings must be documented and corrective actions implemented to resolve the issue promptly. This process safeguards the reliability and accuracy of the traceability system.
Personnel Responsible for Traceability Documentation and Verification
The SOP assigns documenting and verifying traceability records duties to designated production operators and quality control staff. These personnel undergo training to ensure adherence to documentation standards. Continuous verification promotes data accuracy and regulatory compliance.
Retention Duration and Retrieval Process for Traceability Records
Product traceability records must be retained for a minimum specified period, often several years, as outlined in the SOP. During an audit, records are retrieved promptly using a systematic filing and indexing approach. This ensures easy access and demonstrates transparency in manufacturing practices.
