
A SOP Template for Manufacturing Change Management provides a structured framework to document and control changes in the manufacturing process. It ensures consistent evaluation, approval, and implementation of modifications to maintain product quality and regulatory compliance. This template helps streamline communication among teams, minimize errors, and reduce downtime during transitions.
Change request initiation and documentation.

This SOP defines the process for change request initiation and documentation, detailing the steps for identifying, submitting, and recording change requests within a project or organizational framework. It ensures that all proposed changes are properly documented, evaluated, and tracked to maintain project integrity, facilitate communication among stakeholders, and support informed decision-making throughout the change management lifecycle.
Impact assessment and risk analysis.

This SOP details the process of impact assessment and risk analysis, including the identification of potential hazards, evaluation of their likelihood and consequences, analysis of risks associated with projects or operations, and the development of mitigation strategies. The goal is to systematically assess and manage risks to ensure informed decision-making, enhance safety, and promote sustainable outcomes.
Stakeholder review and approval process.

This SOP details the stakeholder review and approval process, outlining the systematic approach for engaging relevant stakeholders in the evaluation, feedback, and formal approval of documents, projects, or proposals. It defines roles and responsibilities, review timelines, feedback integration, and final authorization steps to ensure transparency, accountability, and alignment with organizational objectives.
Change implementation planning and scheduling.

This SOP details the process of change implementation planning and scheduling, covering the identification and assessment of necessary changes, development of a comprehensive change plan, scheduling and coordination of implementation activities, resource allocation, risk management, communication strategies, and post-implementation review. The objective is to ensure changes are executed efficiently, with minimal disruption, and aligned with organizational goals.
Material and inventory evaluation for changes.

This SOP details the process for material and inventory evaluation for changes, covering regular assessment of stock levels, quality control measures, identification of obsolete or excess materials, documentation of inventory adjustments, communication with procurement and production teams, and continuous improvement strategies. The objective is to maintain accurate inventory records, optimize material usage, and ensure timely updating of inventory to support efficient operations and minimize waste.
Update and distribution of revised documentation.
This SOP details the process for the update and distribution of revised documentation, including the steps for reviewing, approving, and making necessary changes to existing documents. It ensures that all revised documents are accurately updated, properly version-controlled, and effectively distributed to relevant personnel. The procedure aims to maintain document integrity, ensure consistent communication, and support compliance with organizational standards and regulatory requirements.
Employee training and communication on changes.
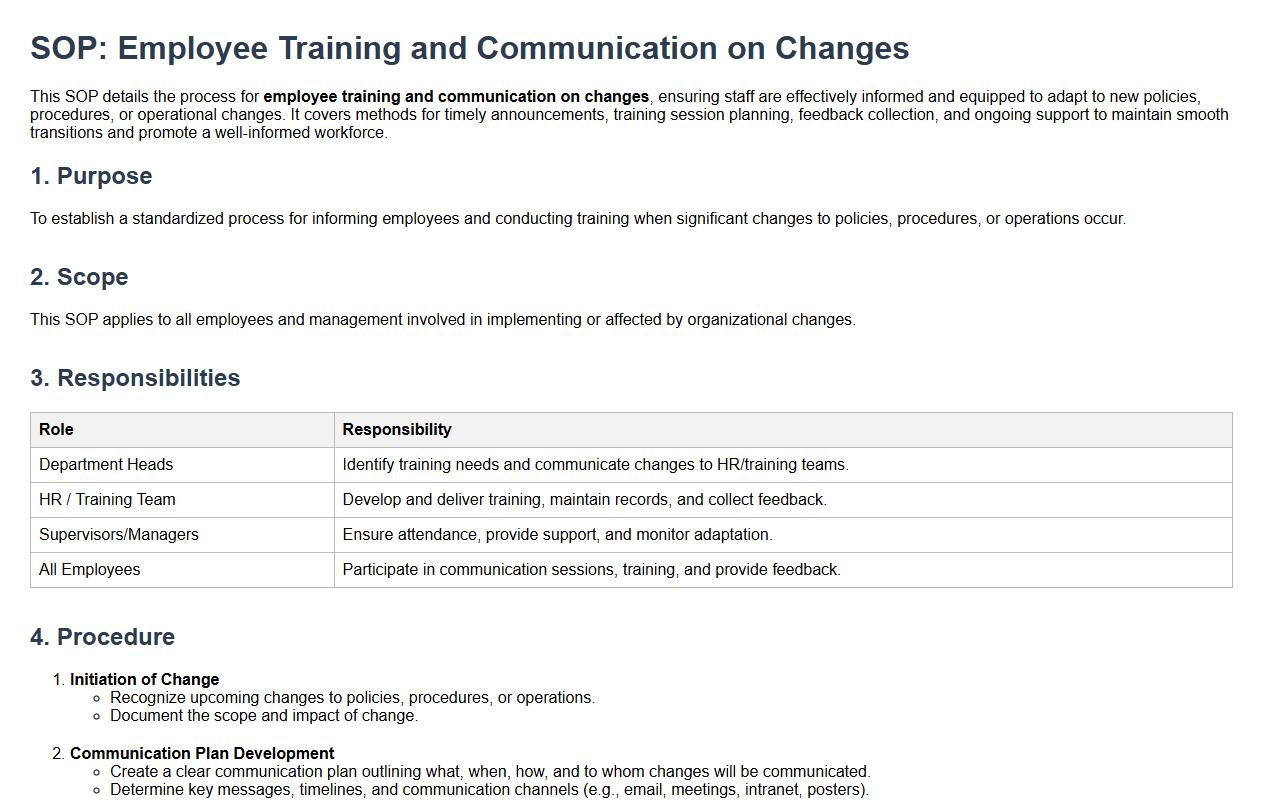
This SOP details the process for employee training and communication on changes, ensuring staff are effectively informed and equipped to adapt to new policies, procedures, or operational changes. It covers methods for timely announcements, training session planning, feedback collection, and ongoing support to maintain smooth transitions and promote a well-informed workforce.
Validation and testing of implemented changes.

This SOP details the process for validation and testing of implemented changes to ensure that all modifications meet predefined requirements and function correctly within the system. It includes steps for creating test plans, executing test cases, documenting results, identifying and managing defects, and obtaining final approval. The goal is to confirm that changes do not adversely affect existing processes and that they deliver the intended improvements with compliance to quality standards.
Change effectiveness monitoring and review.

This SOP details the process for change effectiveness monitoring and review, including the establishment of key performance indicators, data collection methods, and analysis techniques. It covers the periodic assessment of implemented changes, identification of any deviations or issues, and corrective action planning. The objective is to ensure that all changes achieve their intended outcomes, maintain compliance with organizational standards, and support continuous improvement within the business operations.
Archiving records and final change closure.

This SOP details the process of archiving records and final change closure, covering the proper documentation, secure storage, and systematic organization of records to ensure compliance and easy retrieval. It includes steps for verifying the completion of all change-related activities, obtaining necessary approvals, and formally closing the change request. The objective is to maintain accurate records for audit purposes, facilitate knowledge retention, and provide clear documentation of change history across the organization.
What is the primary objective of the Manufacturing Change Management SOP?
The primary objective of the Manufacturing Change Management SOP is to ensure that all changes in the manufacturing process are controlled, documented, and implemented efficiently. It aims to minimize risks associated with changes and maintain product quality and consistency. This SOP sets a standard framework to manage changes systematically within the manufacturing environment.
Which key stakeholders must be notified and involved during a manufacturing change process according to the SOP?
The SOP mandates involvement of key stakeholders such as Quality Assurance, Production, Engineering, and Regulatory Affairs. All these departments must be notified to ensure cross-functional input and risk assessment. Their collaboration ensures the change is feasible, compliant, and aligned with organizational goals.
What are the documented steps for initiating and approving a manufacturing change under this SOP?
The documented steps begin with a formal Change Request Submission, followed by a risk assessment and feasibility study. Next, the change undergoes a structured review by all impacted departments for approval. Finally, documented authorization must be obtained before implementation.
How does the SOP ensure compliance and traceability throughout the manufacturing change process?
The SOP ensures compliance by requiring detailed documentation, tracking, and audit trails for every change. All records of approvals, impact assessments, and implementation steps are maintained for accountability. This traceability supports regulatory inspections and internal quality reviews.
What criteria are used in the SOP to assess the impact and risk of proposed manufacturing changes?
The SOP uses criteria including product quality, regulatory compliance, manufacturing efficiency, and safety to evaluate change impact. Risk assessments consider potential effects on process stability and end-product performance. These criteria guide decision-making to approve, modify, or reject proposed changes.
