
A SOP Template for Manufacturing Supplier Management ensures consistent evaluation, selection, and monitoring of suppliers to maintain quality and reliability in production processes. This template outlines standardized procedures for supplier qualification, performance assessment, and communication protocols. Implementing this SOP helps streamline supplier relationships and mitigate risks in the supply chain.
Supplier qualification and onboarding process.

This SOP details the supplier qualification and onboarding process, encompassing supplier evaluation criteria, documentation requirements, compliance checks, risk assessment, contract negotiation, and system registration. The aim is to ensure that all suppliers meet organizational standards for quality, reliability, and regulatory compliance before integration into the supply chain, thereby fostering efficient and transparent procurement operations.
Supplier performance evaluation and scorecard criteria.
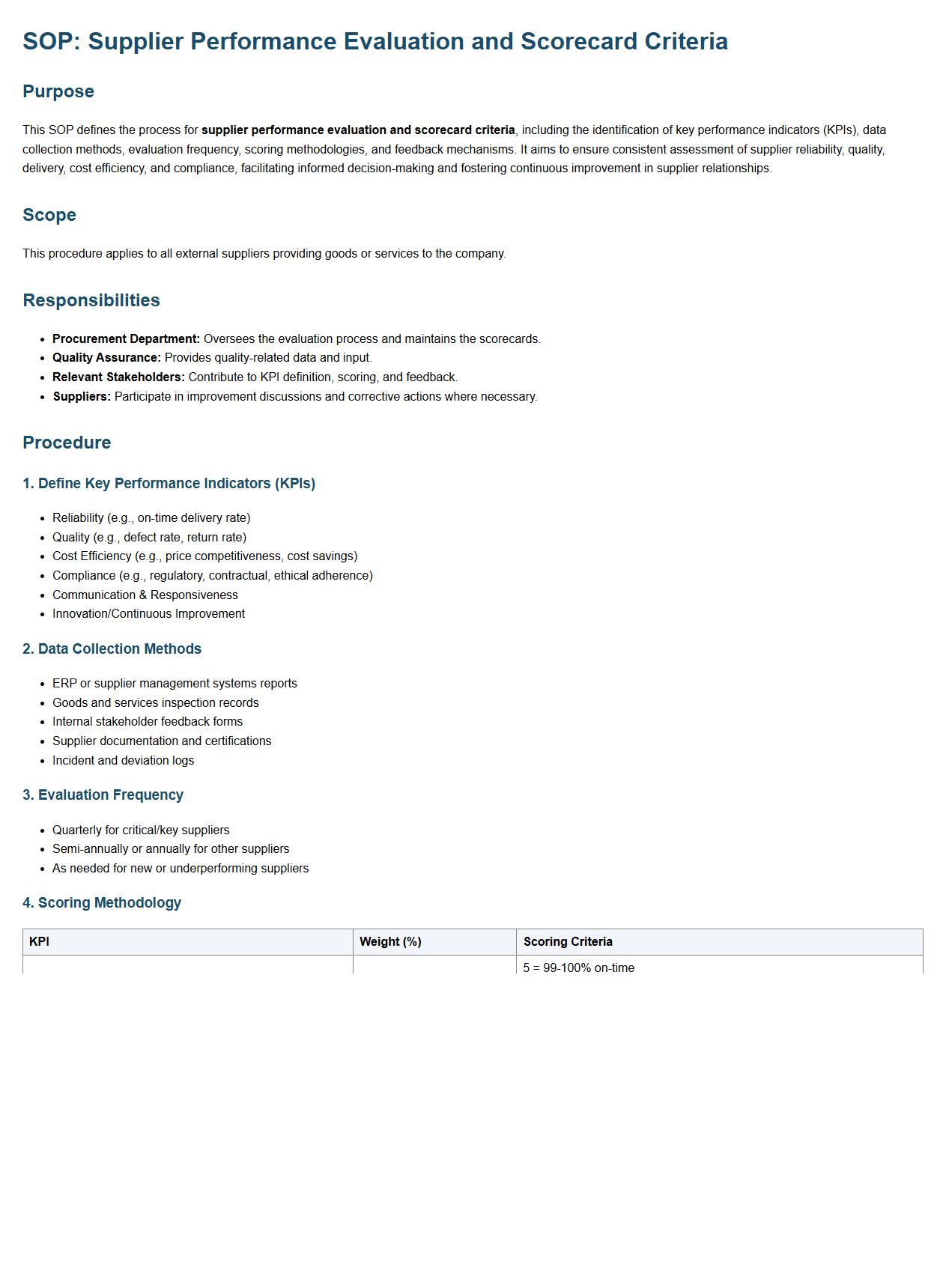
This SOP defines the process for supplier performance evaluation and scorecard criteria, including the identification of key performance indicators (KPIs), data collection methods, evaluation frequency, scoring methodologies, and feedback mechanisms. It aims to ensure consistent assessment of supplier reliability, quality, delivery, cost efficiency, and compliance, facilitating informed decision-making and fostering continuous improvement in supplier relationships.
Quality assurance inspection and acceptance protocols.

This SOP defines quality assurance inspection and acceptance protocols to ensure products meet established standards and specifications. It covers inspection procedures, criteria for acceptance or rejection, documentation requirements, handling of non-conforming materials, and continuous improvement measures. The goal is to maintain product quality, enhance customer satisfaction, and comply with regulatory requirements through systematic and consistent quality assurance practices.
Purchase order issuance and tracking procedures.
This SOP details the purchase order issuance and tracking procedures, including the initiation of purchase requests, approval workflows, purchase order creation and validation, vendor communication protocols, order confirmation, delivery monitoring, and invoice reconciliation. The objective is to ensure accurate, timely, and transparent purchase order processing while maintaining effective vendor relationships and optimizing procurement efficiency.
Supplier communication and escalation channels.
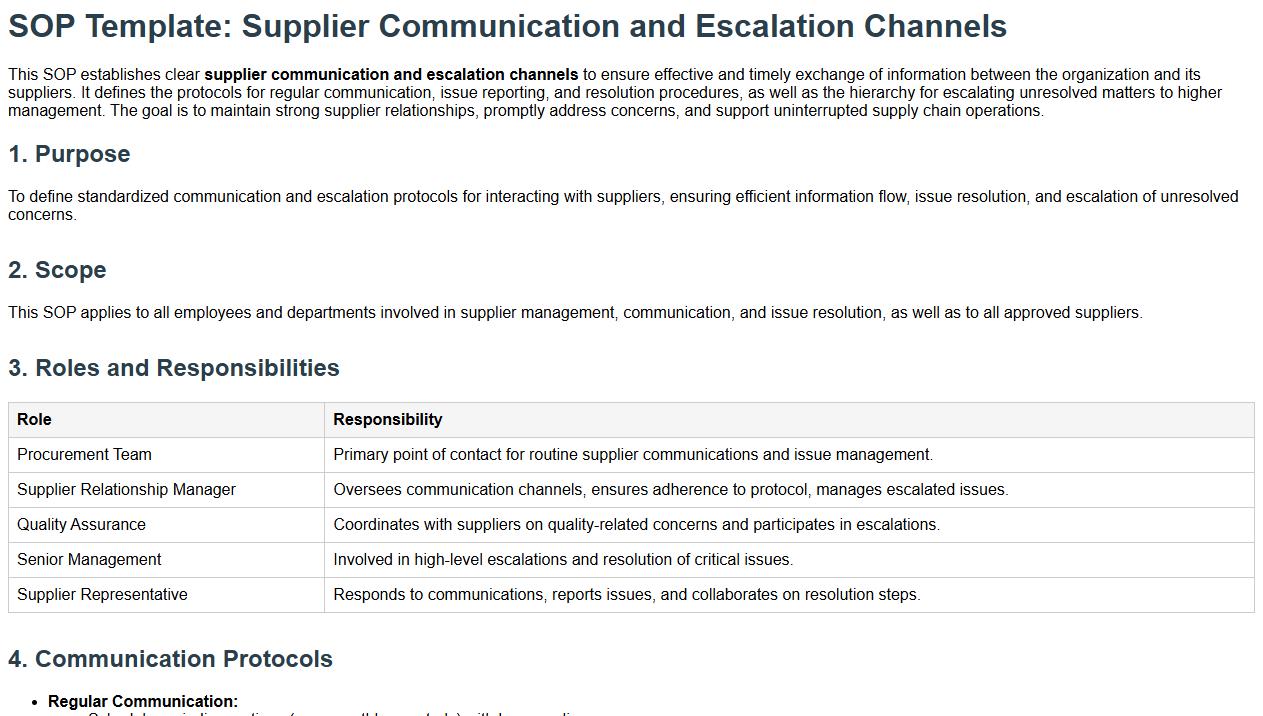
This SOP establishes clear supplier communication and escalation channels to ensure effective and timely exchange of information between the organization and its suppliers. It defines the protocols for regular communication, issue reporting, and resolution procedures, as well as the hierarchy for escalating unresolved matters to higher management. The goal is to maintain strong supplier relationships, promptly address concerns, and support uninterrupted supply chain operations.
Non-conformance and corrective action management.

This SOP details the process for non-conformance and corrective action management, including identification, documentation, evaluation, and resolution of non-conformances. It outlines responsibilities for reporting and investigating issues, implementing corrective actions, monitoring effectiveness, and preventing recurrence to ensure continuous quality improvement and compliance with organizational standards and regulatory requirements.
Change management and notification processes.

This SOP details the change management and notification processes, encompassing the identification, evaluation, approval, implementation, and communication of changes within an organization. It ensures that all modifications to systems, processes, or projects are systematically controlled and documented to minimize risks and maintain operational integrity. The procedures include roles and responsibilities, impact assessments, change scheduling, notification protocols for stakeholders, and post-implementation reviews to verify successful integration and address any issues arising from the changes.
Supplier audit scheduling and execution steps.

This SOP describes the process for supplier audit scheduling and execution steps, including planning audit timelines, notifying suppliers, preparing audit checklists, conducting on-site or remote audits, evaluating supplier compliance, documenting findings, and communicating results. The goal is to ensure supplier quality and compliance with contractual and regulatory requirements through systematic and effective audit procedures.
Document control and record retention requirements.

This SOP details document control and record retention requirements, establishing standardized procedures for managing, storing, and securing organizational documents and records. It includes guidelines for document creation, review, approval, distribution, revision tracking, and archiving to ensure accuracy, confidentiality, and accessibility. The purpose is to maintain compliance with regulatory standards, support operational efficiency, and provide a reliable audit trail for accountability and historical reference.
Supplier contract review and renewal timetable.

This SOP details the process for supplier contract review and renewal timetable, including the scheduling of contract evaluations, criteria for performance assessment, communication protocols with suppliers, documentation requirements, and timelines for renewal negotiations. The goal is to ensure timely contract reviews, maintain supplier quality and compliance, and support business continuity by proactively managing contract renewals.
Criteria for Supplier Approval According to the SOP for Manufacturing Supplier Management
Suppliers must meet specific quality and compliance standards as defined by the SOP for Manufacturing Supplier Management. This includes passing rigorous audits and providing all necessary certifications. Additionally, suppliers are required to demonstrate consistent capacity and capability to meet production demands reliably.
Process for Ongoing Supplier Performance Evaluation and Monitoring
The SOP outlines a continuous performance evaluation system based on key performance indicators such as delivery time, quality metrics, and responsiveness. Regular audits and periodic reviews are integral to maintaining supplier standards. Any deviations trigger corrective actions to ensure ongoing compliance and improvement.
Documented Procedures for Supplier Non-Conformance Handling in the SOP
The SOP details a structured approach to handling supplier non-conformance, including immediate notification, investigation, and root cause analysis. Corrective and preventive actions must be implemented promptly to prevent recurrence. Documentation of all non-conformance events is mandatory for traceability and future reference.
Steps Required for Supplier Qualification and Requalification According to the SOP
The SOP mandates initial supplier qualification through a thorough assessment of quality, capacity, and compliance. Requalification occurs periodically or when significant changes happen within the supplier's operations. Both processes require documented evidence and approval before continued engagement.
Roles and Responsibilities for Managing Supplier Documentation and Communication in the SOP
The SOP clearly defines roles such as the Supplier Manager and Quality Assurance teams responsible for maintaining accurate and up-to-date supplier documentation. Effective communication channels must be established for timely information sharing and issue resolution. Accountability for document control and regular review is emphasized to ensure transparency and compliance.
