
A SOP Template for Raw Material Handling ensures consistent procedures for receiving, inspecting, storing, and managing raw materials in manufacturing or production environments. It outlines clear guidelines to maintain quality control, prevent contamination, and ensure safety throughout the material handling process. Implementing this template helps streamline operations and compliance with industry standards.
Raw material receiving and inspection.

This SOP details the raw material receiving and inspection process, covering the procedures for accepting, verifying, and inspecting incoming raw materials to ensure they meet quality standards and specifications. It includes guidelines for documentation, handling, sampling, and reporting discrepancies to maintain the integrity of the production process and prevent the use of substandard materials.
Verification of supplier documentation and certificates.

This SOP details the process for verification of supplier documentation and certificates, including the review of compliance certificates, authenticity checks, validation of quality standards, and record keeping. It aims to ensure that all supplier documents meet regulatory requirements and company standards to maintain product quality and supplier reliability.
Unloading and storage procedures.

This SOP details unloading and storage procedures to ensure the safe and efficient handling of materials upon arrival. It covers guidelines for unloading equipment, proper handling techniques to prevent damage, inspection protocols, and safe storage practices to maintain product quality and warehouse organization. The procedure aims to minimize risk, optimize space utilization, and maintain inventory accuracy while ensuring worker safety.
Raw material identification and labeling.

This SOP defines raw material identification and labeling procedures to ensure accurate tracking, handling, and traceability of all incoming materials. It includes guidelines for assigning unique identifiers, applying durable labels, verifying material specifications, and maintaining updated records. The objective is to prevent mix-ups, ensure compliance with quality standards, and support efficient inventory management throughout the production process.
Sampling and quality testing of raw materials.

This SOP details the process for sampling and quality testing of raw materials to ensure compliance with quality standards and specifications. It includes guidelines for proper sampling techniques, sample handling and storage, selection of appropriate testing methods, documentation of test results, and corrective actions for non-conforming materials. This procedure aims to maintain consistent product quality and support reliable production outcomes.
Segregation of non-conforming materials.
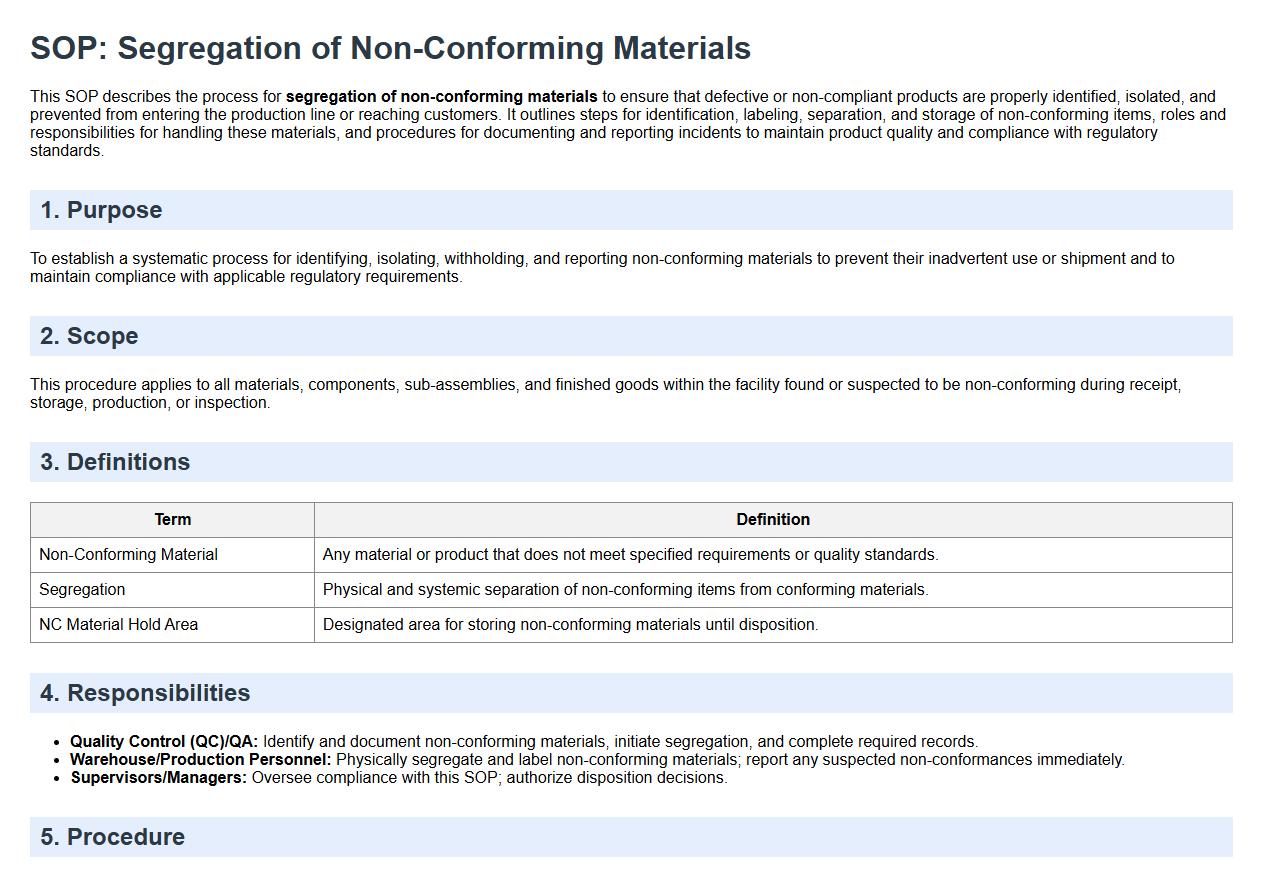
This SOP describes the process for segregation of non-conforming materials to ensure that defective or non-compliant products are properly identified, isolated, and prevented from entering the production line or reaching customers. It outlines steps for identification, labeling, separation, and storage of non-conforming items, roles and responsibilities for handling these materials, and procedures for documenting and reporting incidents to maintain product quality and compliance with regulatory standards.
FIFO (First-In, First-Out) inventory management.

This SOP details FIFO (First-In, First-Out) inventory management procedures, emphasizing the systematic handling of stock to ensure older inventory is used before newer supplies. It includes guidelines for receiving, storing, and rotating products, maintaining accurate inventory records, minimizing waste and spoilage, and optimizing stock levels for efficient operations. The objective is to enhance inventory accuracy, reduce losses, and improve overall supply chain efficiency through disciplined FIFO practices.
Raw material transfer to production area.

This SOP details the raw material transfer to production area process, including receiving inspections, proper handling techniques, documentation requirements, contamination prevention, and safety protocols. The purpose is to ensure the efficient, accurate, and safe movement of raw materials from storage to the production line, maintaining material quality and compliance with operational standards.
Cleaning and sanitization of storage areas.

This SOP details the procedures for cleaning and sanitization of storage areas to maintain hygiene and prevent contamination. It covers the steps for removing debris, selecting appropriate cleaning agents, proper sanitizing techniques, equipment usage, safety precautions, and regular inspection schedules. The objective is to ensure storage areas remain clean, safe, and compliant with health standards to protect stored goods and maintain overall facility cleanliness.
Documentation and record-keeping for traceability.

This SOP details the procedures for documentation and record-keeping for traceability, ensuring accurate and comprehensive recording of product history and movement. It covers proper data entry, maintenance of traceability logs, compliance with regulatory requirements, and secure storage of records. The purpose is to facilitate efficient tracking of products through the supply chain, enhance accountability, and support quality control and recall processes.
What are the critical steps outlined in the SOP for receiving and inspecting raw materials?
The SOP mandates the verification of supplier documentation to ensure authenticity and compliance with specifications. Upon receipt, raw materials undergo a comprehensive visual and quality inspection to detect any defects or contamination. All findings are recorded, and materials are either approved for use or quarantined for further evaluation.
How does the SOP define proper labeling, storage, and segregation of raw materials?
Proper labeling includes clear identification of material name, batch number, and expiry date to prevent confusion and ensure traceability. Raw materials must be stored in designated areas with controlled environmental conditions, maintaining their integrity and quality. Segregation is mandatory to avoid cross-contamination, especially separating approved, quarantined, and rejected materials.
What safety and hygiene protocols must be followed according to the raw material handling SOP?
The SOP requires personnel to adhere to strict personal protective equipment (PPE) usage throughout raw material handling to minimize contamination risks. Regular handwashing and sanitization are emphasized to maintain hygienic operating conditions. Additionally, equipment and storage areas must be cleaned and sanitized following scheduled protocols to ensure safety.
Which documentation and traceability requirements are specified in the SOP for raw material handling?
Every batch of raw material must be documented with a detailed traceability record including supplier information, receipt date, and inspection results. The SOP stresses maintaining comprehensive logs to facilitate quick identification and tracking throughout the production process. This documentation supports compliance with regulatory standards and quality assurance.
What procedures does the SOP recommend for reporting and managing non-conforming raw materials?
Non-conforming raw materials are to be immediately quarantined and clearly labeled to prevent accidental use. The SOP outlines a systematic reporting protocol involving notification to quality control and management teams for timely investigation. Corrective actions are documented, and decisions regarding rework, rejection, or return to supplier are formally recorded.
