
A SOP Template for Manufacturing Plant Cleaning Procedures provides a standardized framework to ensure consistent and thorough cleaning practices. It outlines step-by-step instructions for cleaning equipment, surfaces, and work areas to maintain hygiene and prevent contamination. This template helps improve operational efficiency and compliance with regulatory standards.
Cleaning schedule and frequency guidelines.

This SOP details the cleaning schedule and frequency guidelines necessary for maintaining a clean and hygienic environment. It covers the recommended cleaning intervals for various areas, types of cleaning tasks, and specific frequency requirements based on usage intensity and health standards. The purpose is to ensure consistent cleanliness, prevent contamination, and promote safety and well-being in the workplace or facility.
Designation of cleaning responsibilities per area/staff member.

This SOP details the designation of cleaning responsibilities per area and staff member, ensuring clear allocation of cleaning tasks to maintain hygiene and cleanliness standards. It includes defining specific areas, assigning responsible staff members, outlining cleaning frequency and procedures, monitoring task completion, and establishing accountability to promote an organized and efficient cleaning routine in the workplace.
Required personal protective equipment (PPE) for cleaning tasks.
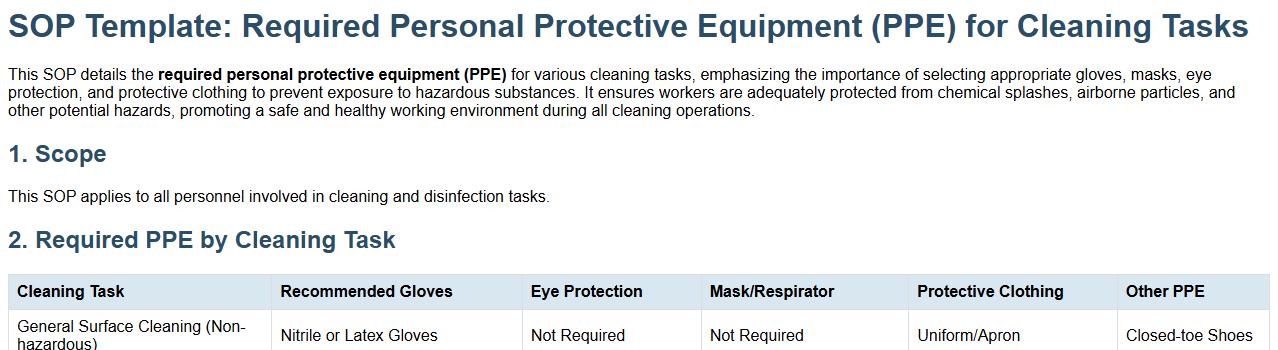
This SOP details the required personal protective equipment (PPE) for various cleaning tasks, emphasizing the importance of selecting appropriate gloves, masks, eye protection, and protective clothing to prevent exposure to hazardous substances. It ensures workers are adequately protected from chemical splashes, airborne particles, and other potential hazards, promoting a safe and healthy working environment during all cleaning operations.
Procedures for cleaning production equipment and machinery.

This SOP details the procedures for cleaning production equipment and machinery, including preparation and safety protocols, cleaning agents and methods, step-by-step cleaning processes, routine maintenance, inspection criteria, and proper disposal of cleaning residues. The aim is to ensure equipment hygiene, prevent contamination, maintain operational efficiency, and comply with industry standards and regulatory requirements.
Surface and floor cleaning protocols.
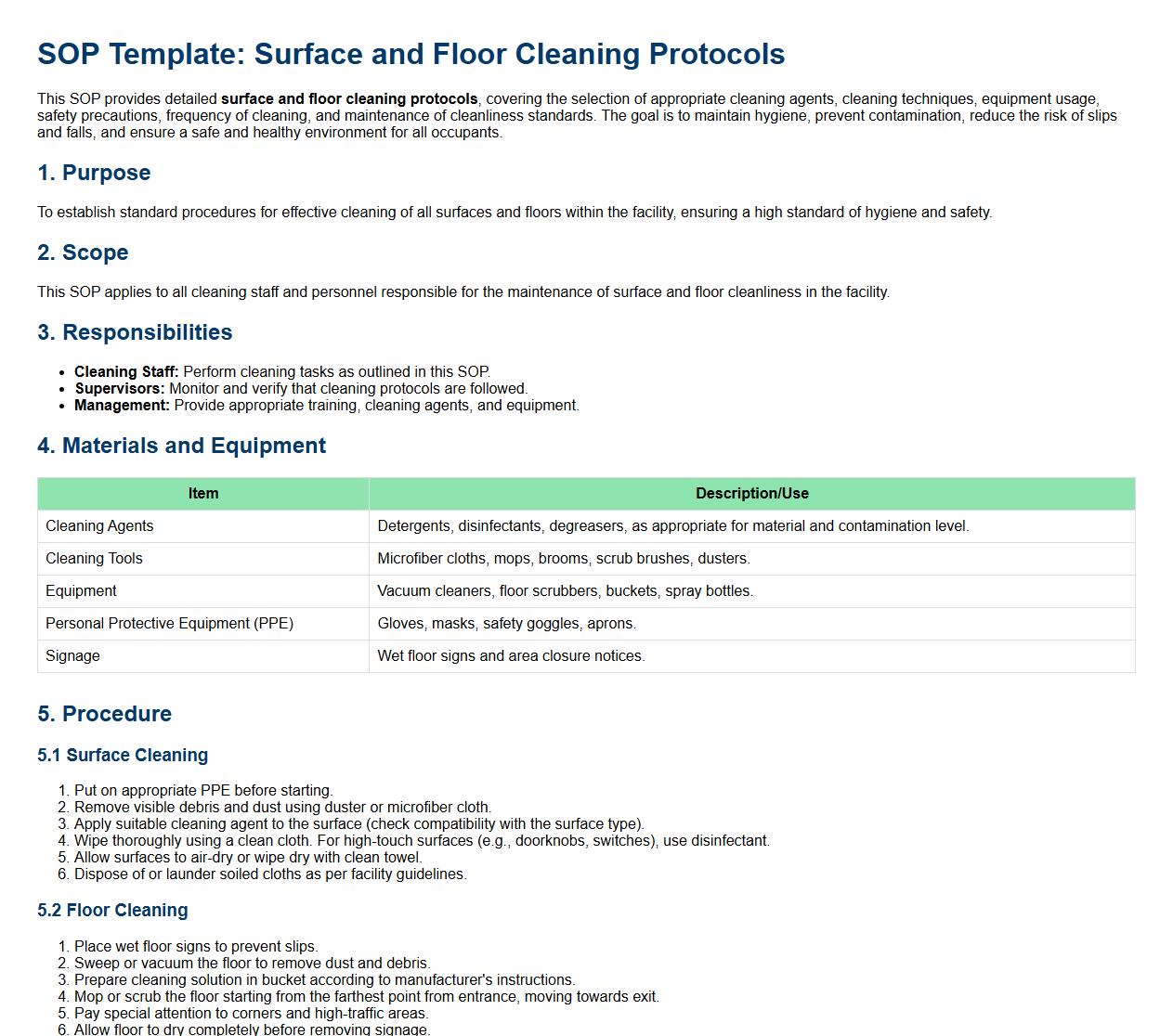
This SOP provides detailed surface and floor cleaning protocols, covering the selection of appropriate cleaning agents, cleaning techniques, equipment usage, safety precautions, frequency of cleaning, and maintenance of cleanliness standards. The goal is to maintain hygiene, prevent contamination, reduce the risk of slips and falls, and ensure a safe and healthy environment for all occupants.
Approved cleaning agents and dilution instructions.

This SOP details the use of approved cleaning agents and dilution instructions to ensure effective and safe cleaning practices. It includes guidelines on selecting appropriate cleaning agents for various surfaces, specific dilution ratios to maintain efficacy while minimizing chemical hazards, proper handling and storage of cleaning chemicals, and procedures for applying diluted solutions to achieve optimal cleanliness and hygiene standards in the workplace.
Waste and chemical disposal procedures.

This SOP details waste and chemical disposal procedures, covering proper identification, segregation, handling, storage, and disposal of hazardous and non-hazardous waste. It ensures compliance with environmental regulations, promotes workplace safety, and minimizes environmental impact by implementing safe disposal methods, spill response protocols, and staff training on chemical management.
Documentation and cleaning checklist completion process.

This SOP details the documentation and cleaning checklist completion process, outlining steps for accurate record-keeping and systematic cleaning verification. It includes instructions for completing cleaning checklists, ensuring compliance with hygiene standards, scheduling regular inspections, and maintaining logs for audit purposes. The process aims to enhance accountability, promote cleanliness, and support quality control across operational areas by standardizing documentation practices and checklist management.
Inspection and verification of cleaning effectiveness.

This SOP details the inspection and verification of cleaning effectiveness, including the procedures for assessing cleanliness, sampling methods, criteria for evaluation, documentation of results, and corrective actions. The purpose is to ensure that all cleaning processes meet established hygiene standards, preventing contamination and maintaining a safe and sanitary environment.
Corrective actions for inadequate cleaning or contamination.

This SOP details the corrective actions for inadequate cleaning or contamination, outlining steps to identify contamination sources, implement proper cleaning protocols, verify cleaning effectiveness, and prevent recurrence. It emphasizes timely response, documentation, staff training, and continuous monitoring to maintain hygiene standards and ensure product safety.
What is the primary objective of the Manufacturing Plant Cleaning SOP?
The primary objective of the Manufacturing Plant Cleaning SOP is to maintain a safe and hygienic environment for production. It ensures that all manufacturing areas meet regulatory standards and prevent contamination. This SOP aims to optimize cleaning processes for operational efficiency and product quality.
Which critical areas are defined as high-priority zones in the cleaning protocol?
High-priority zones in the cleaning protocol include production lines, storage areas for raw materials, and packaging sections. These zones require frequent and thorough cleaning to prevent cross-contamination. Additionally, critical control points such as filters and ventilation systems are highlighted as essential areas for cleanliness.
What are the required cleaning agents and equipment specified for each cleaning task?
The SOP specifies the use of approved disinfectants, detergents, and sanitizers tailored to different surfaces and contamination levels. Equipment such as pressure washers, scrubbers, and microfiber cloths are mandated for effective cleaning. It ensures that all agents and tools comply with safety and environmental regulations.
How are cleaning frequencies and responsibilities assigned in the SOP?
Cleaning frequencies are determined based on the contamination risk and operational schedules, with daily, weekly, and monthly cleaning tasks outlined explicitly. Responsibilities are assigned to designated personnel to guarantee accountability and consistency in execution. This structure supports regular monitoring and adherence to cleaning standards.
What verification and documentation procedures are mandated to ensure SOP compliance?
The SOP mandates regular inspections, cleaning logs, and audit reports to verify adherence to cleaning standards. Documentation must be accurate, timely, and accessible for review by quality control and regulatory bodies. These procedures ensure continuous improvement and risk mitigation in the manufacturing plant.
